General Physics of Atomics
Edited by Jen Moreau
ATOMIC
INTRODUCTION
At the end of 19th century, it was thought that almost all of physics might have been fully explained by mechanics, electromagnetism, and thermodynamics. At that time, only a few minor problem are remained to be resolved or otherwise, physics was believed to be almost complete. The nature of the problem is that there was no form of communications between the field of Mechanics, Electricity, and Magnetism. There were also some effects that lack explanation, but nonetheless, they never affect weaponry, agriculture, and machinery. The problem faced at that time can be stated in three statements
- The speed of light remain constant in all frames of reference
- All the laws of nature were the same in all inertial frame of reference.
- Galilean transformation of absolute time and coordinate.
All these statements contradict one another. One has to be dropped to solve the problem.
THE THEORY OF RELATIVITY
- All inertial frames of reference are equally suitable for the physical phenomena description.
- Speed of light in vacuum is the same for all observers and it is dependent on the source's motion
- The tool for this is a Lorentz transformation of space-time with a set of consequences.
The three big problem with a light that was not resolved are:
- Black body radiation
- Atomic spectra
- Photoelectric effect
BLACK BODY RADIATION
Bodies emit heat radiation that is dependent on their temperature.
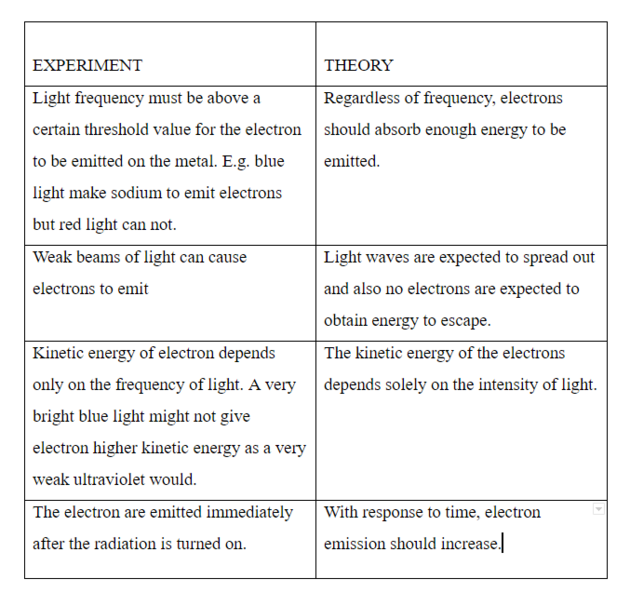
PHOTO ELECTRIC EFFECT
Whenever light shines on metal, the surface of the metal may become positively charged. This is due to the electrons gain energy from the light wave that shines on it and is able to leave the surface of the metal.
SPECTRA
The difference between the spectra emitted by the discharge tubes and the spectrum emitted by a regular light bulb are:
- Spectra emitted by the discharge tubes display distinctive line colors: EMISSION LINE SPECTRA.
- Spectrum of light bulb are a continuous band of colors: CONTINUOUS SPECTRUM
- It is the light emitted due to the filament temperature (Black body radiation).
ABSORPTION SPECTRA
BLACK BODY RADIATION
This was explained firstly by Max Planck. The assumption was that light is emitted in portions called quanta. Energy in each quantum is proportional to its frequency as illustrated in the equation below.
E = h f,
Where h = 6.626 * 10-34 Js
F= the frequency of oscillating atoms.
PHOTONS
PHOTONS PARTICLES AND WAVES
Photons displays properties of waves and particles
- Properties of waves such as frequency, wavelength, polarization, refraction, diffraction, etc
- Properties of a particle such as carrying linear momentum = h/λ, carrying a quantum of energy =hf.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
General Physics of Atomics. (2017). In ScienceAid. Retrieved Apr 24, 2024, from https://scienceaid.net/Atomic
MLA (Modern Language Association) "General Physics of Atomics." ScienceAid, scienceaid.net/Atomic Accessed 24 Apr 2024.
Chicago / Turabian ScienceAid.net. "General Physics of Atomics." Accessed Apr 24, 2024. https://scienceaid.net/Atomic.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Atomic