Electrostatics: The History, The Physics and Applications
Edited by Jen Moreau, Sim, SarMal
If you've recently brushed up on your Ancient Greek or Classic Latin (obscurum per obscurius) you already know the etymological history of the word electricity. The word electricity is directly related to fossilized tree resin, the type of tree resin that made the news this month when scientists discovered a 100 million year old fossilized damsel fly interrupted in the midst of busting a mating groove.
The common term for the resin was Amber, and it continues to be one of the most admired stones around the world. But we have more to thank Amber for than just the fossilized remains that have made up much of our biological and ecological knowledge. The term electricity comes from Classic Latin term electrum derived from the Ancient Greek word elecktron or Amber. Amber is responsible for the very first electrical experiments in 600 B.C. and a catalyst to everything we know about electricity. Those first experiments involved a piece of Amber a bit of friction and opened the world of physics to electrostatics, eventually leading to the knowledge and advancements in electricity that we have today. The word static implies non-movement and electrostatics involves unchanging or stationary electricity. In order to make electricity, electrostatics has to involve movement.
The Evolution of Electrostatics
Unless the power is out most of us in the developed world have a very unconsciously reliant relationship with electricity. We need it, we pay for it and we use it. But the evolution of electricity including our dependence on it is embedded in electrostatics. Long before a pudgy spectacled scientist from Boston went out into a rain storm to self-inflict himself with static electrical charges, Francis Bacon and other scientists were working hard to extract the fundamentals of Electrostatics using the same Amber experiments from centuries before. These experiments continued right up until Benjamin Franklin was able to prove that lightening was electric, static and capable of both positive and negative elements by electrocuting himself. About 40 years later, Charles Augustine de Coulomb was able to prove his law of physics (Coulomb's Law), and it was this law that became the fundamental principles and theories that have established electrostatics.
It wasn't until the very late 1800's that scientists discovered the existence of electrons. This is remarkable when you consider the inventions at the time and those that followed immediately after. Prior to the discovery of electrons, the battery, telephone, iron and the lightbulb had already been discovered while the radio and automobiles were definitely in the stages of concoction. Scientists couldn't label electrons yet, but they were able to use the experiments and science before them to invent, all because of the theories and laws associated with: Electrostatics.
Matter Matters
All matter is made of atoms. And each atom is made up of extremely small particles that include:
- Protons and Neutrons. At the centre of the atom and make up the nucleus and have a positive charge.
- Electrons. Surround or orbit the nucleus and have a negative charge.
With the exception of hydrogen, all matter is made of atoms that follow this rule. The compositions of these atoms in matter are split into two categories:
- 1Conductors have electrons that can detach from the atom and move around. Examples of conductors are:Conductors.Advertisement
- Copper
- Aluminium
- Gold
- Silver
- Mercury
- Bronze
- Lemon Juice
- Water
- 2These are composed of atoms that clutch their electrons tightly. Examples of insulators are:Insulators.
- Rubber
- Glass
- Oil
- Plastic
Positive and Negatives
We already know that protons are positive and electrons are negative. This is key because of the relationships between these two charges. Like most of the dating experiences I've had after my 20's:
- Positive protons can only be attracted to negative electrons.
- Negative electrons can only be attracted to positive protons.
- Negative electrons repel negative electrons.
- Positive protons repel negative electrons.
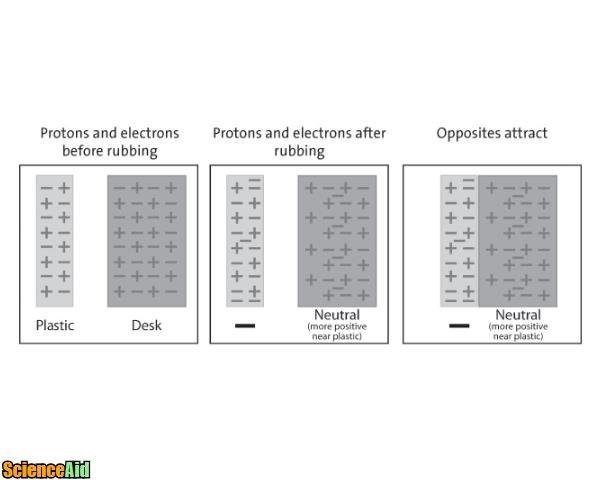
Electricity needs a conductor in order to move. You can't move electricity with an insulator. You've probably been told to stay in your car during a lightening storm. That's because our bodies are conductors. We aren't GREAT conductors, but we are conductors, meaning electricity can pass through our bodies. Everyone has seen this scene on TV or movie; the doctor rubs the paddles and yells CLEAR! then charges the patient's chest. Because our bodies are conductors, the electricity passes through us sending a charge to the heart. The paddles are insulated with plastic so the person performing the defibrillation does not get the charge themselves. Tires on your car work the same way, because their atoms are so tightly bound, the electricity (positive) cannot break through the tightly clutched electrons. The rubber will insulate the static electricity from the ground up, keeping you safe.
If you ever visited your local science museum when you were in middle school, touched the ball and your classmates laughed as your hair went higher and higher, you've witnessed this same principle. This principle however uses...
Electrostatics
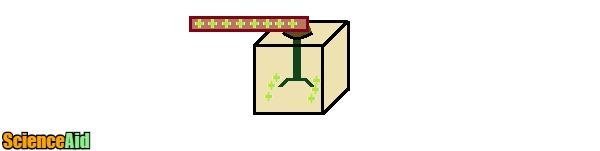
The word static implies non-movement and electrostatics involves unchanging or stationary electricity. In order to make electricity, electrostatics has to involve movement. And just like the Ancient Greeks discovered rubbing silk (insulator) with Amber (conductor) friction is the movement needed. The big ball at the science museum is called a Van de Graaf. The Van de Graaf has a generator inside that works with electrostatic to generate huge amounts of static electricity. You know when you walk quickly across a carpet and then shock yourself grabbing a doorknob? Same principle. Big pieces of rubber move over a big piece of felt, stripping away the felt's electrons. The electrons then move to the metal ball and when a hand is placed on the ball, the electrons repel, running away from each other. They run as far as they can ending at the end of your hair. Your becomes a mess of electrons trying to get away from each other. Genius!
Depending on the positive and negative neutrons moving from an object to another object, they will either repel or attract each other. Attraction creates movement and the movement creates energy.
Experiment #1
- 1Take a plastic grocery bag and cut it into three thin strips.
- 2Assume that the plastic and our skin are neutral and each has the same number of protons and electrons.
- 3You may want to pull the strip up and down through your fingers.Rub a plastic strip quickly with your fingers.
- 4The plastic now has more electrons than protons. This is called a negative charge.Electrons from your skin will jump to the plastic and latch on to the plastic atoms.
- 5Your fingers gave some electrons to the plastic through friction, so now your skin has a positive charge.
- 6
Experiment #2
- 1Take your remaining strips of plastic.
- 2Charge the two pieces of plastic with your fingers.
- 3Slowly bring the two strips together.
- 4Because both strips have extra electrons, they are both negative and negative charges repel.This time, the strips will repel each other.
Experiment #3
This is a simple and fun experiment to demonstrate the attraction of atoms through friction.
- 1Move a balloon quickly across your hair, shirt or plants to give it a static charge.
- 2Turn on the tap and let the water run slowly.
- 3Bring the charged part of the balloon to toward the water.
You will see the stream of water move toward the charged balloon. Watch this quick video
The Physics
Remember Coulomb's law? Coulomb's law states: The magnitude of the force of attraction or repulsion between two stationary point charges (say "q1" and "q2") is directly proportional to the product of these charges, inversely proportional to the square of the distance (r) between them and is directed along the line joining the charges. [1] In other words, as the distance between charges increases, the forces and electric fields decrease. And as we know, the force between the charged objects can be positive or negative. This is dependent on whether the objects are attracted to each other or repelled.
Mathematically then, Coulomb's law is as follows: (1) F ∝ q1q2 (2) F ∝ 1/r2 Combining (1) & (2): F ∝ q1q2/r2 (3) F= K q1q2/r2
- Where 'K' is a constant known as coulomb's constant or electrostatic constant.
- This value of 'K' depends upon the nature of the medium between the charges, and the system of units, in which 'F', 'q' and 'r' are measured.
If the medium between the charges is space or air, then the value of 'K' in SI is 9 x109 Nm2/c2.
- In order to show the dependence of 'K' upon the medium, it is usually expressed in terms of the property of that medium known as permittivity. In the case of free space or air, it is represented by "ε0". The relation between "ε0" and 'K' is given by:
K= 1/ 4π ε0 = 9 x109Nm2/c2.
- So Equation (3) becomes:
F= 1/ 4π ε0 q1q2/r2 : where "ε0" is known as permittivity of free space and has value as Ε0= 8.85 x10-12 c2/ Nm2. If there is an insulator (medium) between the charges, then
- K= 1/ 4π εm
Where "εm" is called the permittivity of the insulating medium.
Unit of Charge
The charge on a single electron is represented by (-e) while the charge of a proton is represented by (+e). Both charges, however, are equal in magnitude.
- For example, e= 1.6 x10-19 Coulombs. This (e) is called the fundamental or SI unit of the charge. Coulomb's law has already been proven in the previous experiments presented, but there is a classic rod experiment generally used to demonstrate Coulomb's law:
- 1Think about a hard rubber rod and a glass rod. Remember which is a conductor and which is an insulator.Experiment 4.
- If you were to rub the rubber rod with fur and rub the glass rod with silk, (moving electrons) then bring the two rods together, the rubber rod will attract the glass rod.
- If two charged rubber rods or two charged glass rods are brought near each other, the force between them will be repulsive, and the rods will move further away from each other.
Much like the plastic strips, this observation shows insulators and conductors are in two different states and depending on the atomic make up and transferring, like charges repel whereas unlike charges attract
Electroscope
The device which is used for the detection and testing of electric charges on a body is called electroscope.
A leaf electroscope consists of a glass case. A conducting rod attaching a disc on its upper end and two thin metallic leaves on its lower end is passed through a cork into the case. The rod will be in suspended position.
If a charged object is brought close to the disc, two types of charges appear; one on the disc and the other on the leaves. The two leaves share the same charge equally and repel each other. If the disc is charged by touching it with the charged object, the disc and the golden leaves acquire the same charge. The greater the amount of charge, the greater will the divergence of the leaves be.
After charging the electroscope, bring the object near the disc of the electroscope. If the charge on the object is the same as that of the electroscope, the divergence of the leaves will increase. It decreases in case of opposite charges.
Electric Field
The space or region surrounding the electric charge (repulsion or attraction) can be felt by another charge, called an electric field or vector. Coulomb's law states that the electric field gets stronger when the charge gets closer.
Electric Field Intensity or Field Strength:
The force per charge of the positive unit, at any point in the electric field, is called the electric field intensity. It is denoted by "E" and is a vector quantity. (1) E= F/ q0 The SI unit of "E" from equation (1) is denoted as N/c The electric field around a charged object can be represented by simply drawing certain lines called electric lines of force. These lines have the following properties.
- 1Each line is drawn such that a line at the field point gives the direction of "E" or "F" at that point.
- 2
- 3
- 4These lines can never cross each other.
- 5
Note: A field which is uniform both in magnitude and direction over a specified region of space is called uniform electric field.
Electrostatic Potential
Electric Potential can be defined as, "the work done per each charge "q0" from a point "A" to a point "B" against the electric field, to have electrostatic equilibrium and in order to move the charge with uniform velocity. The electric potential is denoted by "-"V" and is a scalar quantity. (1) "-"V= WAB/ q0 Consider two points "A" and "B" in the electric field. The electric potential at "A" be V1 and at "B" be V2. If we want to move a charge from point "A" to point "B", the work has to be done against the electric field intensity. This work is stored in the charge in the form of energy known as electrostatic potential.
Potential Difference
The potential difference between two points "A" and "B" can be defined as The energy supplied by a charge as it moves from a point of higher potential to a point of lower potential. VAB= WAB/ q0 VBB-VA = WAB/ q0 Or energy = (VB-VA) q0 The SI Unit of the electric potential is Volt or Joule per Coulomb
Volt
The potential difference between two points in an electric field is said to be one volt if one joule of work is done by a charge of one coulomb when moving from one point to another, 1V= 1J/ 10
Practical Applications of Electrostatics
- 1
- 2Electrostatic induction is used in smoke stacks to clean the air from smoke and other particles. Wire gauze is fixed between two metallic plates in smokestacks. The plates are electrically grounded and the wire is charged positively. This wire gauze produces a negative charge on the inner surface of the plates. An intensive electric field exists between the gauze and the plates. When the air particles exit through the chimneys, they acquire a positive charge by the wire gauze and attracted to the metallic plates. The particles are deposited onto the plate, removing the particles from the outgoing gasses.Dust Extraction.
- 3A photocopier uses electrostatic charge to produce a copy. An image of this page is projected onto a positively charged drum, coated with a conductor. The toner is negatively charged and attracted to the positive parts of the drum. The drum rotates and rolls against the paper, transferring the toner from the drum to the paper making an image of the original.Photocopying.
Questions and Answers
History of electrostatics in physics that who discover this pheneomena?
What is electrostatics and how this term was discover and by whom and what is this pheneomena. Name of any discoverer or physician is not present that term electrostatics by whom it is discovered
If you look under Evolution of Electrostatics in this article you will see the Ancient Greeks as well as Francis Bacon both had a part in the discovery of Electrostatics. The beginning of this article gives a detailed explanation of the evolution of electrostatics and the discoveries leading up to our modern use of electrostatics.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Electrostatics: The History, The Physics and Applications. (2019). In ScienceAid. Retrieved Apr 20, 2024, from https://scienceaid.net/Electrostatics_and_It%27s_Properties
MLA (Modern Language Association) "Electrostatics: The History, The Physics and Applications." ScienceAid, scienceaid.net/Electrostatics_and_It%27s_Properties Accessed 20 Apr 2024.
Chicago / Turabian ScienceAid.net. "Electrostatics: The History, The Physics and Applications." Accessed Apr 20, 2024. https://scienceaid.net/Electrostatics_and_It%27s_Properties.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physics
Recent edits by: Sim, Jen Moreau