Thermodynamics
Edited by TheGuyLoveNY, Jen Moreau, Sharingknowledge, SarMal
Thermodynamics is the branch of physics that deals with heat, energy, and work and involves the transfer of heat into other forms of energy. The Greek word "Therme" translates to heat, while "Dynamics" translates to motion. Etymologically then, thermodynamics is the study of heat in motion. The most famous thermodynamic applications include the internal combustion engine and the refrigerator but thermodynamics surround our everyday modern life.
Heat
Heat is the transfer of kinetic energy from one object to another. These transfers of energy occur through (1) convection (2) conduction or (3) radiation. Since heat is the movement of energy, little energy movement denotes a lower value of heat and termed cold. Heat is denoted by symbol "Q" and has an SI Unit of "Joule". Other units of heat include calories and BTU.
Temperature
Temperature is the degree or intensity of heat in a material or object and the ability of a substance, material or object to transfer heat energy to another source. Temperature is denoted by the symbol "T" and SI Unit of temperature is "k" or Kelvin. Other units include Celsius "C" and Fahrenheit "F".
Kinetic Theory of Gases
The Kinetic Theory of gasses uses particles at the molecular level uses the movement, collision speed and forces of atoms and molecules to describe the properties of gasses. The theory states:
- A finite volume of gas has a large number of molecules.
- The size of the molecule is much smaller than the separation between them.
- The gas molecules are in random motion and may change their direction of motion.
- Due to the random motion, gas molecules collide with each other and the walls of the container.
- The collisions are perfectly elastic.
The pressure exerted by the gas molecules is due to their elastic collision with the walls of the container, which is formulated as:
P = 2/3 No <1/2 MV2> Or P = Constant x <KE> Or P α <KE>
Deviation of Gas Laws
- 1According to Kinetic theory, we have:Boyle's Law:
P = 2/3 N/V < 1/2mv2 > PV = �" N <½ mv2> For a given mass of gas, "N"- No of molecules is constant. Similarly, <KE> is constant. So we may say, PV = Constant Or P α 1/v Which is the statement of "Boyle's Law".
Advertisement - 2Charles Law:From kinetic theory of gasses, we say:
V = �" N/p <½ mV2> For a given mass "N' is constant. If pressure is kept constant then N/P is constant, so: V = Constant <½ mv 2> As <½ mv2> α T, Hence, V α T, or "v/1 = Constant" which is Charles Law
Internal Energy
The sum of all forms of energies of molecules (Kinetic and potential) of a substance is termed as Internal energy. It is denoted by the symbol "You" and SI Unit is "Joule".
In thermodynamics, an ideal gas is considered a working substance. The molecules of ideal gas are mere mass points which exert no forces on each other. So the internal energy of the gas system is generally the translational KE of its molecules.
Changes of Internal Energy
Change in internal energy is represented by "ΔYou". ΔYou is positive when internal energy increases and negative when internal energy decreases. In thermodynamics, internal energy is a function of state (state variable). Consequently, It does not depend on the path but depends on initial and final states of a system.
Work and Heat
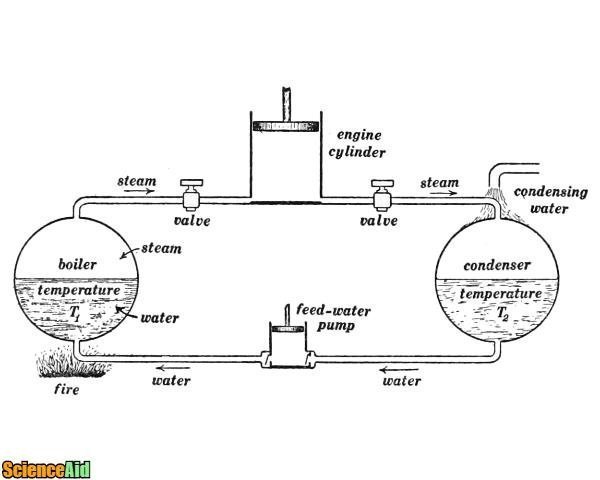
Both work and heat are similar in the degree of energy transfer. This idea was first applied to the steam engine as the transfer involved "Heat in" and "Work out". Both work and heat are defined as positive quantities.
- 1Sign Convention:
- Work done by the system is positive.
- Work done by the system on the environment is positive.
- Heat entering the system is positive.
- Heat leaving the system is negative.
Laws of Thermodynamics
The general principles dealing with heat energy and its transformation into mechanical energy is called the Laws of Thermodynamics. The first law states that the energy of a system is constant and energy cannot be created nor destroyed but only transformed or transported from one source to another.
In any thermodynamic process when heat
ΔQ is added to a system, this energy appears as an increase in internal energy Δ You of system and work done by the system on its "Environment or Surroundings".
When heat is transferred to a system, there is an increase in internal energy and an increase in pressure or change occurs. If at the same time, a substance is allowed to expand then:
The rate of change of heat = Rate of change of internal energy + Work done. ΔQ = (You2 - You1) + Δw But ΔYou = You2 - You1 ΔQ = ΔYou + Δw Which is mathematical form of first law of thermodynamics.
An example of the first law of thermodynamics occurs anything energy is moved from one source to another. For example, imagine an ice cube sitting on the kitchen counter in a container. If you left that ice cube for a few minutes, eventually the ice cube would begin to melt. The ice cube is not losing cold, instead it is gaining heat. The heat is transferred from high temperature objects in its surroundings, increasing the kinetic energy within the container and the ice adding heat and eventually melting the ice. The container is initially warmer than the ice. But the container doesn't gain coldness from the ice, instead, is transfers its increase temperature (heat) to the ice. Mathematically:
-Δw = ΔYou - ΔQ Or ΔQ = ΔYou + Δw
The ability to metabolize energy provides an example of energy conservation since the first law is also known to be another form of the law of conservation of energy. Humans and other animals work when they walk, run or move objects. Work requires energy. Energy is also needed for growth to make new cells and to replace the old cells that have died. The "energy transforming process that occurs with an organism is termed: metabolism". The first law of thermodynamics is applied here as:
ΔYou = ΔQ - Δw.
Applications of the First Law of Thermodynamics
The Isothermal process is a thermodynamic process in which the temperature is maintained or stays the same.
Consider a gas contained in a cylinder made of non-conducting walls, a non-conducting smooth piston, and a conducting base. Once the cylinder is placed on a heat reservoir, at a high temperature (T), the cylinder fulfills the condition for the application of Boyle's law. As such, when a gas expands or compresses isothermally, the product of its pressure and volume during the process remains constant. Where P1, V1, represent the initial pressure and volume. Whereas, P2V2, are pressure and volume after the isothermal change takes place. Then, P1V1 = P2V2
In the case of an ideal gas, the PE associated with its molecules is zero and internal energy of ideal gas depends upon its temperature.
ΔYou = O Then by first law: ΔQ = ΔW + ΔYou ΔQ = ΔW + O ΔQ = ΔW.
Adiabatic process
An adiabatic process is one in which no heat enters or leaves the system, energy is only transferred as work. For an adiabatic process, the prevention of heat flow may be done either by surrounding a system with a thick layer of heat insulating material, such as cork, asbestos, Styrofoam or by performing the transfer so quickly there is not enough time for heat to transfer. Examples of the adiabatic process include:
- Passage of sounds through air.
- Cloud formation in the atmosphere.
There are two types of adiabatic process, as seen from the formula, ΔQ = ΔYou + ΔW under condition, "ΔQ = O", we have, O = ΔYou + ΔW
(1) ΔW = -ΔYou (Adiabatic expansion). (2) ΔYou = - ΔW (Adiabatic compression).
Molar specific Heats of Gasses
The specific heat of gasses is generally stated as molar specific heats. Molar specific heats are the amount of heat required to one mole of the substance through 1K (Kelvin). Molar specific heat is defined in two ways:
- 1Molar specific heat at constant volume:
- 2This is the amount of heat transfer required to raise the temperature of one mole of a gas through one 1K (kelvin) at constant volume.
Mathematically:
ΔQ α Cv ΔT ΔQ = 1CvΔT ΔQ = CvΔT According to first law, ΔQv = ΔW + ΔYou but ΔW = 0, ΔQv = ΔYou ΔYou = Cv ΔT
Molar specific heat at constant pressure is the amount of heat transfer required to raise the temperature of one mole of gas up to one kelvin (1K), at constant pressure. Mathematically:
ΔQp = (1)CpΔT
Δ Qp = Cp ΔT
To prove: Cp - Cv = R
As, ΔYou = Cv ΔT →1
ΔW = PΔV
ΔQp = ΔYou + ΔW
ΔQp = CvΔT + PΔV
ΔQ = CvΔT + RΔT
CpΔT = CvΔT - RΔT
(Cp - Cv)ΔT = RΔT
Cp - Cv = R
A reversible process can be retraced in reverse order, without producing any change in the surrounding. A reversible cycle is a succession of events which bring the system back to its initial condition is called a cycle. A reversible cycle is the one in which all the changes are reversible. However, if a process can not be retraced in the backward direction by reversing the controlling factors, it is an irreversible process.
The Second Law of Thermodynamics
The second law of thermodynamics is defined as, it is impossible to construct such a device that may convert heat, extracted from a single reservoir into work done entirely without any change in the working system. This is also stated by Clausius as it is impossible to cause heat to flow from a cold body to a hot body without the expenditure of external work.
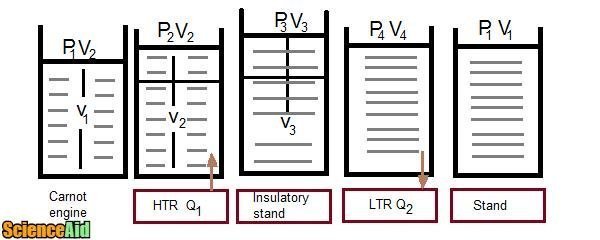
Carnot Engine
In the 1940's "Sadi Carnot" described the ideal engine as free from friction and heat losses, working on the principle that any cyclic heat engine and its cycle is called a Carnot cycle. A Carnot engine consists of a cylinder filled with non-conducting walls, non-conducting smooth piston, and a conducting base. An Ideal gas is used as a working substance.
A Carnot cycle can be explained as:
- The gas is allowed to expand isothermally at temperature T1, absorbing heat Q1 from HTR.
- The gas is allowed to expand adiabatically to temperature T2.
- The gas compressed isothermally at temp T2, rejected heat Q2 to LTR.
- Finally the gas is compressed adiabatically to restore its initial state at temperature T1.
The network done during one cycle equals to the area enclosed by path. ABCDA of the Pv diagram:
δQ = δYou + δW δQ = δW δW = Q1 - Q2
Efficiency
Efficiency = Output (work) / Input(Energy) η = Q1 - Q2 / Q1 = Q1 /Q1 - Q2 / Q1 η = 1 - Q2 / Q1
Percent Efficiency
η % = (1 - Q2/Q1 ) x 100
In terms of temperature,
η % = (1-T2 / T1) x 100
Entropy
"The measure of the disorder of a system is called entropy." Or "Measure of unavailability of energy is called entropy."
This concept was stated by R-clausius in 1856,
Mathematically: δS = δ / T
S.I. Unit of Entropy is Joule per Kelvin (J/K).
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Thermodynamics. (2017). In ScienceAid. Retrieved Apr 19, 2024, from https://scienceaid.net/Thermodynamics
MLA (Modern Language Association) "Thermodynamics." ScienceAid, scienceaid.net/Thermodynamics Accessed 19 Apr 2024.
Chicago / Turabian ScienceAid.net. "Thermodynamics." Accessed Apr 19, 2024. https://scienceaid.net/Thermodynamics.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physics
Recent edits by: Sharingknowledge, Jen Moreau, TheGuyLoveNY