Solubility of salts
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Sim, Sharingknowledge
Soluble and Insoluble Salts
Use the table below to identify which salts are soluble, and which salts are not.
| Soluble | Insoluble |
|---|---|
| All Sodium, Potassium and ammonium | |
| Chlorides | Except silver and lead, chlorides |
| All Ethanoates | |
| Sulphate | Except calcium, barium, and lead sulphates |
| All Nitrates | |
| Sodium, potassium and ammonium carbonates
and hydroxides |
All other carbonates and hydroxides are insoluble |
Preparing Soluble Salts
To prepare soluble salts you must form crystals of them.
- 1If it's not already done, to get the soluble salt, you must react the reactants.React the reactants.Advertisement
- 2Then the solution is passed through filter paper into an evaporating dish to remove excess solid material.Filter.
- 3Now leave the solution. The liquid should evaporate and crystals of the salt will form in the dish.Wait.
Preparing Insoluble Salts
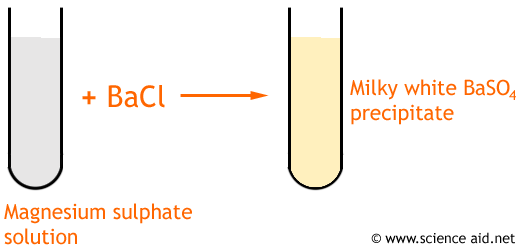
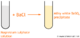
A precipitate is the name given to a solid salt that forms when an insoluble salt is produced from a reaction. For example, with barium chloride and magnesium sulphate...
Barium Chloride + Magnesium Sulphate ==>> Barium Sulphate + Magnesium Chloride BaCl2 + MgSO4 ==>> BaSO4 + MgCl2.
You will see a white precipitate of barium sulphate ...
To get a pure, dry sample of Barium Sulphate it must be:
- 1Filtered.
- 2Washed with distilled water,
- 3Left to allow the excess water to evaporate.
Questions and Answers
Please I need to get the solublities of mgcl2,cacl2,alcl3,nacl,li in acid and base?
Please help me out to get those answers because ii have searched the internet. Help out wit the questions please and I would really appreciate
The molar solubility represented by s is the concentration of a dissolved salt in an ionic solution. To find 's', you can use the solubility constant Ksp. For example, for the dissociation of MgCl2
*MgCl2 → Mg++ + 2Cl-
s 2s
Ksp = [s][2s]2
Ksp= 4s3
s3=Ksp/4
s = 3√(Ksp/4)
Write a method to explain how to obtain a pure dry sample of sodium chloride?
Give a method.. This is a test, therefore, I require a bit of help. To help find the solution and method, you must have a brain.
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Solubility of salts. (2017). In ScienceAid. Retrieved Apr 25, 2024, from https://scienceaid.net/chemistry/applied/solubilityofsalts.html
MLA (Modern Language Association) "Solubility of salts." ScienceAid, scienceaid.net/chemistry/applied/solubilityofsalts.html Accessed 25 Apr 2024.
Chicago / Turabian ScienceAid.net. "Solubility of salts." Accessed Apr 25, 2024. https://scienceaid.net/chemistry/applied/solubilityofsalts.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: Sim, Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)