Ammonia and Haber Process
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), SmartyPants, StephWrites and 2 others
The Haber Process
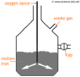
The Haber Process is the process by which ammonia (NH3) is produced. The equation for this reaction is:
The symbol you see in the middle means it is a reversible reaction, so the product can decompose back into the reactants. Optimum conditions must be selected to achieve the greatest yield. When the forward and backward reactions are the same, it is said to be in a state of dynamic equilibrium.
The position of this dynamic equilibrium can be moved forward by changing the conditions of the reaction. This follows Le Chatelier's Principle which says changes to a system in equilibrium will move it in an opposite direction.
| Condition | Effect |
|---|---|
| Pressure | Increasing this will improve the yield because the forward reaction reduces pressure. However, increasing the pressure too far is impractical and becomes too expensive because special instruments must be used to withstand the forces. |
| Temperature | A higher yield can be obtained by using a low temperature since the forward reaction produces heat, but this also will make the reaction slower, and less profitable, so a temperature of about 450°C is optimal. |
| Catalyst | The Haber Process makes use of iron to speed up the reaction - but this doesn't improve the yield. To discover more about reaction rates, see rates of reaction. |
The conditions of the Haber Process must be finely balanced to reach a combination of highest yield, and fastest reaction, this is very important because getting this right will make sure this industrial process is as profitable as possible.
Other Uses
Ammonia is commonly used as a general house hold cleaner. The solutions of ammonia used as a surface cleaner is NH3 in water or ammonia hydroxide. Because ammonia produces a streak free shine, it is commonly used to clean glass, ovens and kitchen floors. However, in recent years most household cleaners are made without the use of ammonia because many people find the smell unpleasant the corresponding fumes off putting.
Ammonia is also commonly used in fertilizers because of the nitrogen it releases into the soils. In North America, ammonia is often directly applied by commercial application tanks to the soil. In science, ammonia is used as ammonium salts (ammonium nitrate, sulfate, and phosphates. Ammonia is also used as an ingredient in commercially used explosives such as nitroglycerin.
Fertilizers
Ammonia is used in the production of fertilizers. A nitrogenous fertilizer is manufactured by neutralizing ammonia with nitric acid.
nitric acid + ammonia ==>> ammonium nitrate HNO3 (aq) + NH3 (aq) ==>> NH4NO3 (aq)
These fertilizers promote the growth of plants, but, as the adage goes, for every action there is a reaction - this can cause problems.
- 1Nitrates are very soluble and are washed out of the soil by rain, where they may travel to a lake, river, pond etc.Advertisement
- 2The fertilizer causes a large boom in plant growth, but this boom is followed by a fall in plant life.
- 3All of the dead material from the fall in plant life, causes decay by bacteria that use up the oxygen in the water, The results of this are aquatic animals that rely on oxygen in the water will die.
Other Uses
The main use of ammonium is as a fertilizer, however, it is also commonly used as a household cleanser. Because ammonia offers a streak-less shine, it is used to clean mirrors, glass and stainless steel. In science, ammonia is used as ammonium salts such as ammonium sulfate, nitrates, and phosphates. Ammonia is also used as an ingredient in commercially made explosives such as nitroglycerin.
Questions and Answers
Why does the Haber process have to be carried out at such high temperatures?
How does the temperature affect the rate of reaction?
During a Haber process for ammonia synthesis, According to Le Chatelier's Principle: if the temperature employed is too high, equilibrium moves in the direction of the opposite reaction, and less ammonia is created.
According to this Principle, to get as much ammonia as possible, the temperature should be low. However, a lower temperature also means a slower process. Since this process is used by manufacturers trying to produce as much product as possible, their aim is not to achieve an equilibrium mixture which takes years for the reaction to reach equilibrium but to get as much product as they can in a short amount of time.
So a compromise temperature must be defined to obtain the greater yield of product: slightly low to obtain a decent yield of ammonia but high enough to obtain a fair rate of reaction. 400 - 450°C has been found to be a compromise temperature that allows producing big amounts of ammonia in a short time.
Although that temperature is apparently high to human standards, in industrial processes, higher temperatures can be reached. So even if it seems like very high temperatures, they are barely high enough on that industrial temperature spectrum.
How can I explain, in one sentence, why the Haber process does not follow Le Chatalier's principle?
How can I explain, in one sentence, why the Haber process does not follow Le Chatalier's principle? I am trying to be concise in a powerpoint presentation to a group of non-scientists. Thermodynamics & P-chem are not my forte, and it has been 30 years since I had those classes.
This answer might be tricky since Haber process itself does follow Le Chatalier´s Principle, however, in the production process for ammonia most manufacturers are in search of yield to obtain as much product as they can in the less time instead of trying to reach an equilibrium mixture.
- 1In this way, either the forward or the backward reaction will be favored, thus producing a higher or a lower yield of product.The Principle pronounces that if the conditions of a closed system at equilibrium experience change, then the equilibrium position will shift to the direction that will oppose this change.
- 2Since this process goal is to produce as much product as possible, their aim is not to achieve an equilibrium mixture which takes years for the reaction to reach equilibrium but to get as much product as they can in a short amount of time.Haber process for ammonia production uses Le Chatalier´s Principle to determine yield, not equilibrium.
It might seem as Haber process for ammonia production does not follow Le Chevalier's Principle since its goal is not to look for the conditions to obtain equilibrium, but it does follow the Principle to determine the conditions to obtain a better yield.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Ammonia and Haber Process. (2018). In ScienceAid. Retrieved Apr 19, 2024, from https://scienceaid.net/chemistry/applied/haber.html
MLA (Modern Language Association) "Ammonia and Haber Process." ScienceAid, scienceaid.net/chemistry/applied/haber.html Accessed 19 Apr 2024.
Chicago / Turabian ScienceAid.net. "Ammonia and Haber Process." Accessed Apr 19, 2024. https://scienceaid.net/chemistry/applied/haber.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: SarMal, StephWrites, SmartyPants