Testing for Cations
Edited by Jamie (ScienceAid Editor), Meforcollege, Jen Moreau, SmartyPants and 5 others
- 1 What is Cation Testing?
- 2 Flame Test
- 3 Sodium Hydroxide Test for Cations
-
4 Questions and Answers
- 4.1 Why do cations form and how do we tell by the color?
- 4.2 I am looking for the Cation test which uses HCL, are you able to tell me what it is?
- 4.3 What is the theory behind how cation and anion tests work?
- 4.4 NaOH + Ca(2+). Will the reaction fprm a precipitation?
- 4.5 Problem with distinguishing between lead and aluminum both of which are colorless solutions?
- 4.6 I want to know the advantages and disadvantages of using dilute acid to test for carbonate?
- 4.7 Testing for cations using different solutions of NaOH, Ammonia and Sodium Carbonate?
- 4.8 Can sodium hydroxide to identify other metal cations than the ones mentioned on the table?
- 4.9 How to identify washing soda from caustic soda by using vinegar and lemon juice?
- 4.10 Explain the scientific principles behind the tests used to identify chemicals?
- 4.11 Is there any easy way in memorizing the tests for cations and anions?
- 4.12 What is the cations in flame for NaCl. Sr(NO3)2,KNO3?
- 4.13 I'm studying for the chemistry IGCSE and there is a point I have no clue about?
- 4.14 Is there a trend with adding NaOH to Period 3 elements?
- 5 Referencing this Article
- 6 Comments
What is Cation Testing?
Testing for cations is a test used in chemistry to identify metal or metal ions (cations) found in compounds. There are two types of tests used in chemistry to test for cations.
- 1The Flame test involves exposing the compound to a flame and identifying the compound by the flame color produced. When the compound is heated, the electrons move to energy levels that are higher. This movement makes the ions energetically unstable and they move back into the previous energy join. As they move back, the ions release light energy.on the type of ions, this light energy is different and produces differing colors of flame.Flame Test.Advertisement
- 2This test uses sodium hydroxide or aqueous ammonia to test and identify metal ions by the precipitation formed. Sodium Hydroxide or Aqueous Ammonia is added to the solution being tested and the color of precipitation formed allows for identification of the compound.Sodium Hydroxide Test.
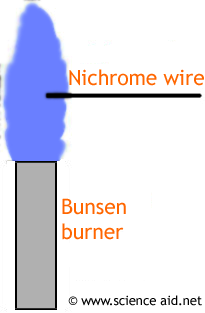
Flame Test
A flame test can be used to identify a compound using a flame. The procedure is as follows:
- 1Use a clean nichrome wire.
- 2Heat the nichrome wire.
- 3Dip it in the hydrochloric acid.
- 4Dip the wire into the compound you are testing so a small blob is collected on the wire.
- 5Put the tip of the wire into a flame and see what color it turns.
These are the colors you will see for different ions:
| Ion | Color |
|---|---|
| Sodium (Na+) | Orange-yellow |
| Potassium (K+) | Lilac |
| Calcium (Ca2+) | Brick-red |
| Copper (Cu2+) | Green |
Sodium Hydroxide Test for Cations
Add several drops of sodium hydroxide (NaOH) solution to the solution being tested. If a colored precipitate is formed then stop and find out what the cation is. If white precipitate forms then continue to add NaOH to it and observe whether the precipitate dissolves.
| Cation | Precipitate color | Further results |
|---|---|---|
| Aluminium (Al3+) | White | Precipitate dissolves as more NaOH is added to the solution |
| Calcium (Ca2+) | White | Precipitate will not dissolve in the NaOH solution |
| Copper (Cu2+) | Pale blue | none |
| Iron(II) (Fe2+) | Pale green | none |
| Iron(III) (Fe3+) | Red-brown | none |
The ionic equation for these reactions are all very similar, here is an example it with Aluminium:
Al3+(aq) + 3OH- ==>> Al(OH)3(s)
In order to test any other ionic equations, to change the number of OH- ions so that it balances with the oxidation state of the metal anion. E.g. Iron (II) would need two OH- whereas Iron(III) needs 3.
Questions and Answers
Why do cations form and how do we tell by the color?
How do we know by the color of the precipitate for which cation it is, is it the bonds
First I would like to explain why cations are formed. Before that, you have to understand the atomic structure. Atomic structure is similar to that of the solar system. In place of the sun, there is a nucleus where neutrons (neutral charged) and protons (positively charged) are present. Electrons orbits around the nucleus like planets. Shell is like layers covering the nucleus. In each shell, there will be a certain number of electrons. In the first shell there will be two electrons and in the second shell there 8 and in the third again there is 8 and it goes on as the atomic number increases. A shell is complete when all the electrons are filled.
An atomic number is a number of electrons present in an atom. A number of an electron will always be equal to the number of protons hence an atom does not have charge. When an atom loses its electron it becomes positively charged and when it gains an electron it will be negatively charged. An atom can lose or gain only its electrons, proton number will always remain the same.
Now consider sodium (Na) atom. Na has the atomic number of Na is 11. hence its atomic structure is 2,8,1. It has a lone electron in the outer shell. In general, atoms have the tendency to make the outer shell complete. If Na loses one electron its outer shell will be complete. When an electron is lost, it proton number will be increased by 1, since electron and protons are equal. When the number of the proton is increased than electron it charges increase to positive. Hence cations are formed.
Similarly, in chlorine atom, Cl needs one electron to make in outer shell complete, hence it gains electron easily. When it gains an electron, its charge reduces to -1 due to the presence of one extra electron
For your second question, you should know about transition metal in the periodic table. Transition metals are colored which is its fundamental property.
I am looking for the Cation test which uses HCL, are you able to tell me what it is?
Hi Jamie, I am looking for the Cation test which uses HCL, are you able to tell me what it is?. Thanks. It will help a bunch
As I explained in the previous answer, color is the inherited property of transition metal. If you are looking for the reaction which will produce color, you can perform displacement reaction with any transition metal.
Take the example of Fe
Feso4 + hcl=fecl3 + h2so4
ferrous (fe2+) is converted into ferric (fe3+)
What is the theory behind how cation and anion tests work?
What is the theory behind cation and anion tests and what are some chemical equations used to show how it works? Thanks. The article does not go into detail into how the cation and anion tests work on a chemical level. I have tried: Googling more and looking in textbooks. Looking back at coursework. I think it was caused by Difficulty finding enough information. Not many people have told about how it works in theory
Colors are produced by the transition metal, which is its inherent properties.
The other cause for producing colors occurs at the atomic level. For example, when you heat a metal, electrons are excited due to gaining of energy. When they return to the normal state, gained energy is release out. This released energy sometimes will be in the visible range of light spectrum. Colors are produced according to that of the wavelength of energy released.
NaOH + Ca(2+). Will the reaction fprm a precipitation?
Precipitation question. We must show the balanced equation. Different reactions are used. NaOH + Ca(2+), balanced equation.
Here is an example of Balanced Equation
Ca(NO3)2(aq) + 2 NAOH(aq) = Ca(OH)2(s) + 2 NANO3(aq)
Problem with distinguishing between lead and aluminum both of which are colorless solutions?
How do you differentiate between aluminum and lead ions using the sodium hydroxide test. Both give you white precipitates that redissolve when more sodium hydroxide is added. Can you use a flame test to distinguish the two? Flame testing is generally used for alkali metals such as Lead. The flame test used on lead will produce a gray/white flame.
I want to know the advantages and disadvantages of using dilute acid to test for carbonate?
It's a chemistry related question, most about inorganic unknowns. I have searched and searched but I can't find it, I need the answers asap please because it's not included in the article
When using dilute acid to test for carbonate or carbonate materials, hydrochloric acid is added to the rock or mineral. The release of carbon dioxide and the forming of bubbles denote the presence of carbonate materials. The main disadvantage of this test is the variance in the bubble formation - it can happen very fast and being very identifiable or it may occur slowly and need magnification to observe. The ubiquitous nature of calcite can also lead to confusing results with this test.
Testing for cations using different solutions of NaOH, Ammonia and Sodium Carbonate?
Can the cations in a solution be identified by using another solution. for example, the cations were identified using NaOH but can they be identified using aqueous ammonia and sodium carbonate
The solution used to test for cation largely depends on the type of component you are testing for. There are three types of solutions for testing: Sodium Hydroxide, Ammonium Hydroxide, Sodium Carbonate. The solution used is dependent on the cation or component you are testing for. The flame test is generally used when testing alkali metals, while transition metals form differing precipitations when a solution is added.
Can sodium hydroxide to identify other metal cations than the ones mentioned on the table?
Yes. Sodium Hydroxide and Ammonia can be used to identify transition metals such as Aluminium, Calcium, Copper, Iron (II), Iron (III), Chromium, Manganese, Cobalt, Nickel, and Zinc.
How to identify washing soda from caustic soda by using vinegar and lemon juice?
Two bottles of soda were left on a shelf in a garage their labels were half torn so the only word soda was still intact. one was washing soda and the other was caustic soda, how would test to know which one is which?
To identify the bottles, you want to create a reaction using the vinegar or lemon juice. When you add vinegar to washing soda (NaC03) the reaction is as follows:
Na2 CO3 + 2 CH3COOH = 2 CH3COONa + CO2 + H2O.
The reaction will appear as water. But, if you add vinegar or lemon juice to baking soda (NaHC03) the reaction will produce carbon dioxide: NaHCO3 + HC2H3O2 = NaC2H3O2 + H2O + CO2. This reaction will produce discernible bubbles as the carbon dioxide escapes. Baking soda and vinegar are often used in model volcanoes to create the appearance of a volcanic eruption.
In short, you would be able to identify the difference between the washing soda and the baking soda because the baking soda will create noticeable bubbling while the washing soda creates water.
Explain the scientific principles behind the tests used to identify chemicals?
Please see the newly added section "What is Cation Testing" in this article.
Is there any easy way in memorizing the tests for cations and anions?
I find it quite confusing to remember the cation and anion tests in chemistry and was wondering if there was an easier way The different colors, precipitation involved in cation testing and anion testing can be difficult to remember. See some of the diagrams in the article to help and consider mnemonics as a tool to remember the tests. You might also want to consider doing as many cation and anion tests as possible - it is often much easier to remember experiences.
See more questions like this: Why are aqueous hydroxide ions used to test for cations?
What is the cations in flame for NaCl. Sr(NO3)2,KNO3?
Please help me know what will happen on the flame test for cations. I need cations in flame for Nacl, Sr(NO3)2, Cu(NO3)2, KNO3. Thank you so much. It says that obtain a few crystals of table salt on the tip of a clean scoopula and place the tip in the flame of a bunsen burner under the hood. Elements have characteristic colors when placed in a flame. Test strontium nitrate, copper (II) nitrate and potassium nitrate in the same manner as the sodium chloride to see the color of flame produced by each of these cations. Some of these compounds are used in firework productions. Record your observation. It just shows the procedure of how to do it, but the results are not in there. They used other cations in the experiment so that is why the answers that I am looking for is not in there. I have tried: Nothing actually. I am afraid to touch or start a bunsen burner because I have a trauma of it. I think it was caused by: Because I am afraid of fire. I had burned myself before through flames so it is not easy for me to work with a Bunsen Burner.
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
I'm studying for the chemistry IGCSE and there is a point I have no clue about?
The syllabus says the following (and I've got no clue of what to look for or how to find the info or what it even means):. . . 1 Use the following tests to identify:. aqueous cations:. • ammonium, copper(II), iron(II), iron(III). and zinc by means of aqueous sodium. hydroxide and aqueous ammonia as. appropriate (formulae of complex ions. are not required). anions:. • carbonate by means of dilute acid and. then limewater. • chloride by means of aqueous silver. nitrate under acidic conditions. • nitrate by reduction with aluminium. • sulfate by means of aqueous barium ions. under acidic conditions. gases:. • ammonia by means of damp red litmus. paper. • carbon dioxide by means of limewater. • chlorine by means of damp litmus paper. • hydrogen by means of a lighted splint. • oxygen by means of a glowing splint. I don't know what to look for so I don't know how to apply the article to it
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
Is there a trend with adding NaOH to Period 3 elements?
I have recently done the AQA 2017 A-level Chemistry Paper 1 past paper (Inorganic and Physical) to revise. In the paper, I was asked to identify one simple test-tube reaction to distinguish between aqueous solutions of magnesium chloride, MgCl2, and aluminium chloride, AlCl3. The answer was to add NaOH and see how both produce a white precipitate but only the AlCl3's precipitate would dissolve in excess NaOH. As I have never really come across this before, I was wondering if you could explain the trend with adding NaOH to Period 3 chlorides and other metal ions please, such as if they produce different coloured precipitates or if they react differently and why. For example, why does the AlCl3 precipitate dissolve with excess? I would very much like to know the role of the OH-. Thank you very much in advance. It involves the testing of metal ions not shown in the tables and further explanation. I have tried: My Chemistry A-level textbook and other websites, I feel this is a great place to find the answer. I think it was caused by: Having to apply reactions to other scenarios and apply trends to different groups
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Testing for Cations. (2020). In ScienceAid. Retrieved Apr 20, 2024, from https://scienceaid.net/chemistry/applied/testcations.html
MLA (Modern Language Association) "Testing for Cations." ScienceAid, scienceaid.net/chemistry/applied/testcations.html Accessed 20 Apr 2024.
Chicago / Turabian ScienceAid.net. "Testing for Cations." Accessed Apr 20, 2024. https://scienceaid.net/chemistry/applied/testcations.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: sofitad, Albie, SarMal