Bonding
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Jen Moreau, Sim
Three Kinds of Bonding
Important note, electronegativity is the ability of an atom to attract electrons. (see periodicity). Imagine that an element with a higher electronegativity has more pulling power in a 'tug-of-war', so it is able to pull electrons with less electronegativity toward itself.
Ionic Bonding
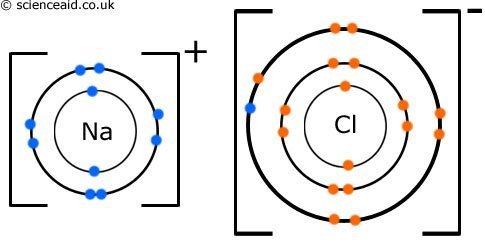
Bonding occurs between metals and non-metals like our friend sodium chloride. The reaction can be represented as follows, showing the electron arrangement, shown in the full form below:
Electrons are transferred from a metal to a non-metal, forming two oppositely charged ions. Both have the electronic structure of a nobel gas. The ionic bond is the electrical attraction between these two ions. The ions do not work on a 2D basis. They can attract oppositely charged ions in all directions, thus making a huge ionic lattice. In NaCl, each cation is surrounded by 6 anions and vice versa. However, depending on the size of the ions, more or less can surround it.
The properties of giant ionic structures are as follows.
| Property | Reason |
|---|---|
| High melting and boiling points | The electrostatic forces between ions are very strong and a lot of energy is required to break up the lattice. |
| Very poor electrical conductivity as a solid. But very good when molten or dissolved. | As a solid, the conductivity is very poor because the electrons are all in a fixed position and cannot transfer charge.
However, when molten or dissolved, the electrons are free to move. When dissolved the lattice separates into its charged ions: mobile charge carriers. |
| Soluble in polar solvents (e.g. water) | The lattice is broken up by the polarity of the solvent, this leaves the ions suspended in the solvent. |
In reality, no compound has 100% ionic character where there is a complete transfer of electrons. Between the two extremes of ionic and covalent bonding, is a world in between known as polarization. The degree of polarization in ionic compounds is determined by the charge density of the cation (positive), and how high the negative charge of the anion is. The charge density is high if the cation is small but at the same time, it has a high positive charge.
As you go across period II, the charge size increases and so does the ion charge. So the charge density increases until Al which has a very high charge density. As you can see in the above representation, the Al3+ will distort the electron cloud more and thus have a greater polarization effect.
Covalent Bonding
Covalent bonds make molecules, and form between non-metals. It is formed when electrons share a pair of electrons rather than transfer them. A good example of covalent bonding is water:
Covalent bonds have the following properties...
| Property | Reason |
|---|---|
| Low melting and boiling points | The inter-molecular (see below) bonds between molecules are weak. |
| Poor electrical and thermal conductivity. | The electrons are in a fixed position and so cannot move to transfer charge. |
Covalent bonds can be categorized as either polar or non-polar. In a non - polar molecule, the electrons are shared equally because the atoms have similar electronegativities. In a polar molecule, there are quite different electronegativities. Water (above) is polar. The oxygen molecule is more electronegative so the electrons are unequally shared and shifted toward it. This makes oxygen slightly negative. This is represented by the symbol δ- delta minus, and the hydrogen atoms are therefore both δ+. This molecule is a permanent dipole. There are also dative covalent bonds (or co-ordinate bonds), when the shared pair of electrons comes from just one of the atoms. It is represented in diagrams using an arrow, showing the direction the electron pair is denoted.
Metallic Bonding
As the name suggests, this type of bonding is between metals. In this type of bonding many positive metal ions occupy a fixed position in a lattice (a bit like ionic). Its outer electron energy level becomes delocalised, creating what is known as a sea of electrons since they are not fixed and free to move throughout the lattice.
The metallic bond is the electrostatic attraction between the cations and delocalized electrons. This bond is very strong. And below are its properties and explanations for why they are so.
| Property | Reason |
|---|---|
| High melting and boiling points | There are very strong forces between the cations and electrons so a lot of energy is required to break the bonds. |
| Very good electrical and thermal conductivity. | The delocalized electrons are able to move freely in the 'sea'. These mobile electrons are therefore able to carry charge or heat energy. |
| Poor Solubility | The electrostatic attraction between ions and electrons is too strong to be broken by the solvent. |
Questions and Answers
Why are electrons in metallic bonding referred to as "delocalized"?
Thanks for helping understand this. Can you also tell me the properties of metallic bonding and how atomic and electron arrangement reflects the property? I couldn't find the answers in the website. the picture are a bit to big. I have tried: Yes I saw other websites. I think it was caused by: I don't know
The reason the metallic bonding is referred to as 'delocalized' is because of its free electrons that are circulating around the positively charged nuclei. Electrons, for any element, are located in the electronic cloud of the atom and orbit around it. In metals, some of the electrons at the s and p orbitals delocalize and move closer to the positive nucleus. This characteristic allows the metals to react more freely with other metals to form alloys and to conduct electricity in a better way.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Bonding. (2017). In ScienceAid. Retrieved Apr 25, 2024, from https://scienceaid.net/chemistry/fundamental/bonding.html
MLA (Modern Language Association) "Bonding." ScienceAid, scienceaid.net/chemistry/fundamental/bonding.html Accessed 25 Apr 2024.
Chicago / Turabian ScienceAid.net. "Bonding." Accessed Apr 25, 2024. https://scienceaid.net/chemistry/fundamental/bonding.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Fundamental
Recent edits by: Jen Moreau, Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)