Rates of Reaction
Edited by Jamie (ScienceAid Editor), Jeff Moreau, Jen Moreau, Sim and 3 others
Everything around us is made of matter, and all matter consists of chemicals. In order for matter to change or to make new matter, these chemicals need to interact with each other. These are termed chemical reactions and they occur constantly. It is almost impossible to mentally fathom the number of chemical reactions that occur inside you, around you and outside of you every nanosecond. Chemical reactions are endless and continual and include everything from burning wood, your body's digestive and conversion of food, starting a car, driving a car, washing a car, manufacturing, fireworks even baking cupcakes ALL of these involved chemical reactions. These chemical reactions fluctuate in the rate at which they occur. Depending on the number of chemicals involved in the reaction, the physical state and speed of the molecular movement and the complexity of the reaction, some reactions are immediate and the effects are seen instantly, while other reactions can take days, weeks even years to demonstrate the reaction.
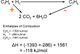
The type of chemical reaction, the change of matter and the effect of the reaction deeply depends on the reaction rate. The reaction rate is the speed (how fast or how slow) two things interacting respond to the interaction.
Collision Theory
Collision Theory was first propositioned by Willian Lewis in 1918 and demonstrated chemical reaction occurrence and differences in the rates of chemical reactions. Collision Theory postulates that in order for a successful chemical reaction to occur, the particles must collide with sufficient energy to break or change bonds and create new bonds. Imagine an illusory bunch of little particles (representing molecules) whizzing about, and bouncing into walls as well as into each other. Those particles that bump into each other with enough energy will bond. The amount of energy released when two particles collide into each other (as opposed to the stationary wall), enables the release of a sufficient amount of energy to transfer, modifying the bonds or breaking the bonds and creating new bonds. This illustrates a successful chemical reaction through collision theory. The amount of energy they need to react is the activation energy which is the minimum energy required for a reaction to occur. The activation energy required depends on several factors including:
- The number of chemicals/bonds involved in the reaction.
- The physical state (molecular speed of movement).
- Intricacy or complexity of the reaction required.
- Collision Force and Speed (Concentration).
- Pressure, Order and Temperature.
Maxwell-Boltzmann Distribution
If you refer back to the imaginary example of particles whizzing around in an enclosed room, the molecules contained are all traveling at different speeds (velocities), with differing forces and at different magnitudes. The air molecules surrounding the particles are also traveling differently with different temperatures. The Maxwell-Boltzmann distribution is named after er James Clerk Maxwell and Ludwig Boltzmann who created the curve to explain the relationships between particules moving within a container (or enclosed room) that do not interact with each other except to exchanged energy and momentum through collision. The curve uses temperature to measure the speed of molecules in a gas at a certain temperature. The Maxwell-Boltzmann distribution curve is as follows:
- On the y (vertical) axis is the number or fraction of molecules
- On the x (horizontal) axis is the amount of energy.
- The curve shows the proportion of particles that have a particular energy.
- The area underneath the curve show the total number of molecules with energy.
Marked on a particular point on the graph is the activation energy, while the area shaded in green shows the fraction of particles that have energy more than or equal to the activation energy. These are the particles that will react. The blue curve (T1) is at a lower temperature and T2 at a higher. This graph demonstrates that the heat in a reaction is increased when the number of particles with the activation energy is greater, in turn increasing the rate of reaction.
Factors Affecting Rate
There are factors other than temperature that also affects the rate of reaction. These are concentration, surface area and catalysts.
The graph above shows how the concentration of the products and reagents varies over the course of a reaction: as the concentration of the reactants drops, the concentration of the products climbs since they are directly related.
The surface area affects the rate of reaction. The top line shows the reaction when a powder is used. The rate of reaction is greater because there are more available particles to collide with. When a substance that is denser and has tightly packed molecules is used the reaction rate is less because not as many particles are available.
A catalyst is a bit different. Rather than making more collisions, a catalyst makes successful collisions more likely by lowering the activation energy without itself being used up. If you think of it in terms of the Maxwell-Boltzmann distribution then the Ea is moved to the left and more particles are in the shaded region.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Rates of Reaction. (2017). In ScienceAid. Retrieved Apr 20, 2024, from https://scienceaid.net/chemistry/physical/reactionrate.html
MLA (Modern Language Association) "Rates of Reaction." ScienceAid, scienceaid.net/chemistry/physical/reactionrate.html Accessed 20 Apr 2024.
Chicago / Turabian ScienceAid.net. "Rates of Reaction." Accessed Apr 20, 2024. https://scienceaid.net/chemistry/physical/reactionrate.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physical
Recent edits by: vcdanht, SarMal, Sim