Group 2, Alkaline Earth Metals
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Jen Moreau, SarMal and 1 other
Trends
Very quickly we shall go over the trends in properties of Group 2 elements using the below table.
| Atomic Radius | The atomic radii increase down the group. This is because new electron shells are added to the atom, making it larger. |
| 1stionisation energy | The first ionization energies decrease down the group, this is because there are more electron shielding and a greater distance of the outermost electrons from the nucleus. |
| Electronegativity | The electronegativity decrease down the group because the nuclear charge has less effect on the outermost electrons, so the atom will give them up more readily. |
Reactions in Water
When added to water, the first alkaline earth metal, (Beryllium), is totally nonreactive, and doesn't even react with steam. Then as you move down the group, the reactions become increasingly vigorous.
As an example, the following reaction takes place between magnesium and water, an alkali earth metal hydroxide and hydrogen gas is produced. Magnesium can be substituted for any Group 2 metal, however.
Mg(s) + H2O(l) ® Mg(OH)2 (aq) + H2
When magnesium is reacted with steam, it is even more vigorous, and instead of a hydroxide, an oxide is produced as well as hydrogen gas.
Mg(s) + H2O(g) ® MgO(s) + H2 (g)
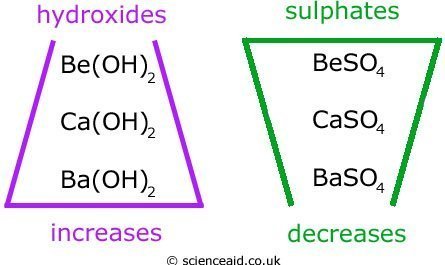
Solubility
Here we shall look at the solubilities of the hydroxides and sulfates of Group 2 metals.
Solubility is the maximum amount a substance will dissolve in a given solvent. It is measured in either, grams or moles per 100g of water.
The trends of solubility for hydroxides and sulfates are as follows:
Magnesium hydroxide (Mg(OH)2) is said to be sparingly soluble because it does not dissolve in very well and Be(OH)2 and BaSO4 are insoluble.
The chemical test for a sulphate is to add Barium Chloride. If positive, the solution will go milky. This means the precipitate barium sulfate has been formed; which, as you can see from the solubility table above, this is insoluble.
Beryllium is Different
Beryllium differs from its brothers and sisters in Group 2 in that it usually forms covalent bonds, but unlike other covalent molecules, it is soluble in organic solvents and a poor conductor when molten. Another strange feature is that it is amphoteric. This means it has the properties of both an acid and a base. The two reactions below show this:
Be(OH)2 (s) + H2SO4 (aq) ® BeS4 (aq) + 2H2O(l)
Be(OH)2 (s) + 2NaOH(aq) ® Na2Be(OH)4 (aq)
Questions and Answers
Please help me to understand the solubility trend of group 2 salt trend?
Solubility is the maximum amount a substance will dissolve in a given solvent. It is measured in either, grams or moles per 100g of water. As salt is a sulfate, the solubility differs depending on the type of salt and whether the salt is hydrated or not. Magnesium sulfate and calcium sulfate (both salts) are considered insoluble unless they are hydrated.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Group 2, Alkaline Earth Metals. (2017). In ScienceAid. Retrieved Apr 25, 2024, from https://scienceaid.net/chemistry/fundamental/group2.html
MLA (Modern Language Association) "Group 2, Alkaline Earth Metals." ScienceAid, scienceaid.net/chemistry/fundamental/group2.html Accessed 25 Apr 2024.
Chicago / Turabian ScienceAid.net. "Group 2, Alkaline Earth Metals." Accessed Apr 25, 2024. https://scienceaid.net/chemistry/fundamental/group2.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Fundamental
Recent edits by: SarMal, Jen Moreau, Taylor (ScienceAid Editor)