Amines and Amino Acids
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), gopi vamsi, Jen Moreau and 1 other
Properties of Amines
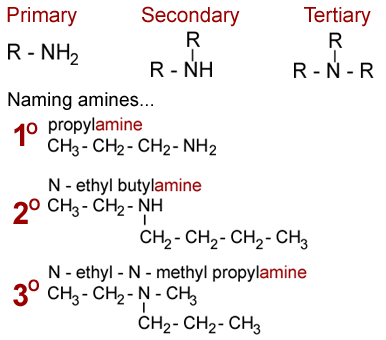
Amines have the functional group N. We have the primary, secondary, and tertiary amines; their structures are outlined in the diagram below.
Amines are basic because of a lone pair of electrons in the nitrogen atom, this means it can accept protons (hydrogen ions) and is therefore a Brønsted-Lowry base. The strength of the base is related to the availability of the lone pair and hence the electron density. Ammonia (an amine with H as R) is a weaker base than primary amines, because the alkyl group pushes electrons towards the lone pair.
However, aromatic amines, are less basic than ammonia, because the delocalized electrons in the benzene ring reduce the electron density.
Making Amines
Amines can be made by the nucleophilic substitution of ammonia and amines with haloalkanes, the reaction mechanism is as follows.
However, as discussed above, primary amines have the nitrogen lone pair even more available than ammonia. This means that further substitution will occur to produce secondary, tertiary amines and quaternary ammonium salts. These are used as cationic surfactants which have a variety of applications, including their use in fabric softeners, paints and laxatives.
Primary amines can also be made by reacting a haloalkane with cyanide. This substitutes the Br to produce a nitrile: which has the functional group -C≡N. This is then reduced to an amine. Note how the carbon chain increases by one in this method.
RBr + CN- ® RC≡N + Br- RC≡N + 2H2 ® RCH2NH2
Aromatic amines can be produced by the reduction of C6H6NO2, by using a Nickel catalyst.
C6H6NO2 + 3H2 ® C6H6NH2 + 2H2O
Properties of Amino Acids
Below is the structure of an amino acid, where the R represents a side chain. Notice how the amino acid has two functional groups: the amine and carboxylic acid group.
The way that amino acids are named, is that the carbon in the carboxylic group is carbon 1. Alpha (α) amino acids have the amine on the second carbon; β (beta) amino acids have the amine on the third carbon and so on. However, all naturally occurring amino acids (those that are used in the body in proteins) are α-amino acids.
The amino acid is charged differently according to the pH conditions it's in. If it is in [[chemistry/physical/acidbase.html|acidic] conditions there is an abundance of H+ so the nitrogen lone pair accepts a proton. However, if it is in alkaline conditions the H+ in the COOH is donated.
At a certain pH (specific to each amino acid), the isoelectric point is reached, and a zwitterion is formed. This has both a positive charge on the nitrogen and a negative charge on the oxygen.
Proteins
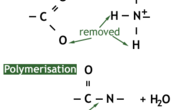
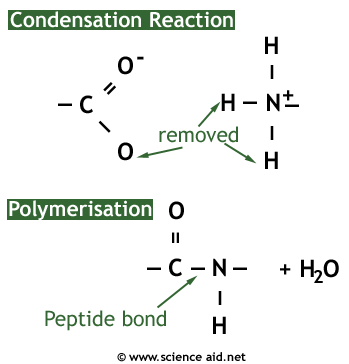
A protein is a naturally occurring polymer of amino acids. They are formed by a condensation reaction which forms a peptide bond between two amino acids, and water as a byproduct.
The reverse of the condensation reaction is hydrolysis, which requires water and will break a protein into its constituent amino acids.
The 3D structure of proteins is what gives them their biological properties. This is determined by hydrogen bonding. So the different sequences of amino acids produce atoms in different places and hence, a unique shape.
Questions and Answers
How many amino acids are primary amines? Secondary amines? Names?
We just learned a week ago about proteins and enzymes but am kind of lost as to how to answer this question, it seems a little too broad of a question. It briefly talks about their structure but I am not sure how you can tell.
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
I want to know about primary secondary and tertiary amines?
How can we differentiate them with examples. How the carboxylic acids are different from them?
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Amines and Amino Acids. (2017). In ScienceAid. Retrieved Apr 25, 2024, from https://scienceaid.net/chemistry/organic/amines.html
MLA (Modern Language Association) "Amines and Amino Acids." ScienceAid, scienceaid.net/chemistry/organic/amines.html Accessed 25 Apr 2024.
Chicago / Turabian ScienceAid.net. "Amines and Amino Acids." Accessed Apr 25, 2024. https://scienceaid.net/chemistry/organic/amines.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Organic
Recent edits by: Jen Moreau, gopi vamsi, Taylor (ScienceAid Editor)