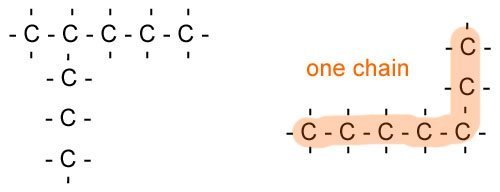Nomenclature
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
Nomenclature is defined as a system of giving things names. In the case of Organic Chemistry, this obviously applies to all of the different compounds, and more importantly, their isomers. The way compounds are named is standardized across the world by IUPAC (International Union of Pure and Applied Chemistry), so scientists everywhere know what each other is talking about.
Types of Formulae
This is something covered here, but they are important for this unit and we've got an extra one.
- 1This give the simplest ratio of element atoms to each other.Empirical Formula.Advertisement
- 2This gives the actual number of element atoms in a molecule.Molecular Formula.
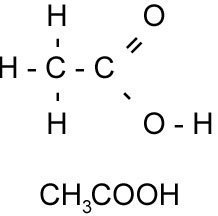
- 3This shows the atoms present and all the bonds between them.Structural Formula.
The above example shows the two types of structural formula we have. The pictorial representation shows every single bond; but drawing lots of these out is difficult. So you can write an abbreviated formula which shows the order in which the bits of the molecule appear.
How Many Carbons?
Organic Chemistry is all about carbon, and so an important part of the name lets you know how many there are. The table below gives the bits of word and how many carbon atoms it means there are.
| Carbons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Name | Meth- | Eth- | Prop- | But- | Pent- | Hex- | Hept- | Oct- | Non- | Dec |
For example, an alkane with formula C7H16 will be called Heptane
Naming Rules
Before you are presented with a huge, terrifying and confusing table here are some explanations to clear some things up.
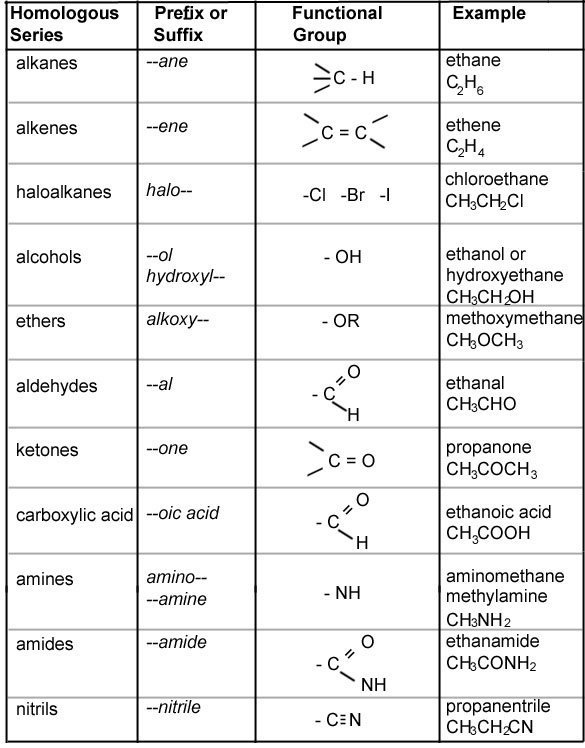
- 1This is an atom or group of atoms that gives the entire molecule its properties i.e. the reactive bit.Functional Group.
- 2A family of molecules all with the same functional group, but increasing numbers of carbon atoms is called a homologous series. They can be represented by a general formula which uses algebra to tell you how to work out the molecular formula of any molecule in the family.Homologous Series.
The information above, should give you enough information to understand the table below.
Further Naming Rules
And if all that wasn't enough, there are some more rules regarding naming. These rules allow you to know the precise structure of the molecule.
- The functional group position is shown by a number in the name. For example, 2-chloropentane means the chlorine atom comes off of the second carbon atom in the branch. And But-2-ene means the double bond is between the second and third carbons. This number is always the smallest possible. You wouldn't call it 4-chloropentane because it is the same as 2-chloropentane just rotated.
- We use the words di (two) tri and tetra (four), are used to say how many functional groups there are; for example: dichloromethane is CH2Cl2.
- In terms of how you write it; numbers and letters are separated by a hyphen; numbers and numbers are separated by commas. And when there are several functional groups you put everything in alphabetical order.
- This final rule is quite important. Sometimes, you might have a compound that has a chain coming off of it, especially with alkanes and alkenes, like the one shown below.
The different bits coming off of the hydrocarbon on the left (imagine there are Hs coming off of the lines), are all named according to the rules. So the chain is 4-methyl (it is on the fourth carbon in the main branch and 1 carbon large) the main chain has 7 carbons so it is heptane. All together the molecule is called 4-methylheptane.
The chain on the right doesn't have any side chains but is actually a continuous alkane called heptane, you can tell which is the side chain by drawing a line along it with your pencil. If you can go through all the carbons without taking it off and going back on yourself, then it is on chain.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Nomenclature. (2017). In ScienceAid. Retrieved Apr 19, 2024, from https://scienceaid.net/chemistry/organic/nomenclature.html
MLA (Modern Language Association) "Nomenclature." ScienceAid, scienceaid.net/chemistry/organic/nomenclature.html Accessed 19 Apr 2024.
Chicago / Turabian ScienceAid.net. "Nomenclature." Accessed Apr 19, 2024. https://scienceaid.net/chemistry/organic/nomenclature.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Organic
Recent edits by: Jamie (ScienceAid Editor)