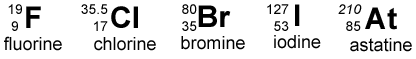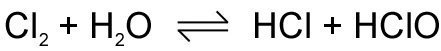Group: VII: Halogens, Halides and Chlorine
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), SmartyPants, MaxScience
Properties of Halogens
- 1The electronegativity of halogens decrease down the group. Fluorine is very reactive and is the most electronegative element.Electronegativity.Advertisement
- 2A further physical property is the halogen's boiling point, which increase down Group 7. This is because the atoms get bigger and so the Van der Waals forces get larger as well.Boiling Point.
- 3And now a chemical property. The oxidizing power of the halogens decreases from Fluorine to Iodine. This property can be demonstrated by displacement reactions.This means if halogen ions are dissolved in water. If a halogen higher up the group is added; this higher halogen will replace it. For example, when chlorine is added to Br-(aq), the following reaction takes place.Oxidization.
2Br-(aq) + Cl2 ® 2Cl-(aq) + Br2
Halides
A halide is simply the ion of a halogen. Their ability to reduce increases down the group from F to I. This ability is shown in the reactions of NaX (where X is a halogen) with sulphuric acid, or as chemists like to call it: H2SO4. The table below shows the results of the reactions of NaX with sulphuric acid.
| NaX | Observations | Products |
|---|---|---|
| Fluorine | Steamy fumes | HF |
| Chlorine | Steamy fumes | HCl |
| Bromine | Steamy fume
Colourless gas Brown fumes |
HBr
SO2 Br2 |
| Iodine | Steamy fumes
Colourless gas Yellow solid Rotten eggs smell A black solid a purple fumes |
HI
SO2 S H2S I2 |
What this demonstrates is that more products are formed with the sodium halides further down the group, therefore reducing the ability of these halides is greater at the bottom of the group.
How to Test for Halides
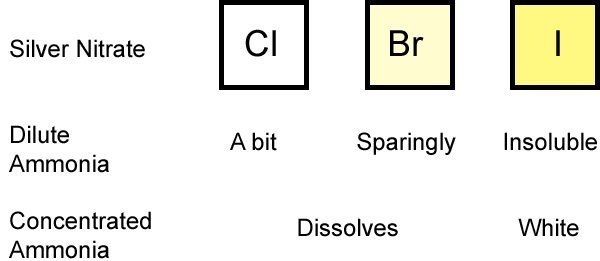
Now - how to test for the presence of the different halides. A classic test is to add silver nitrate, and it produces a precipitate of different colors depending on the halide. However, as you can see below, the difference in color is not very distinct. Therefore, we add another substance: ammonia. First, a few drops of dilute ammonia are added to the precipitate to see how much it dissolves. Chlorine will dissolve quite well. Bromine will dissolve sparingly and Iodine not at all. Then we add concentrated ammonia and the precipitate in both the chloride and bromide solutions will dissolve and the iodide precipitate turns white. The diagram below outlines this testing procedure.
The ionic equation for this reaction is as follows (using Br as the halide).
Cl-(aq) + Ag+(aq) ® AgCl(s)
Salt Formation
Halogens will react with metals to form salts. For example, if chlorine gas is passed over a heated iron wire, you will see a brown solid upon cooling.
iron + chlorine ® iron (III) chloride 2Fe(s) + 3Cl2 (g) ® 2FeCl2 (s)
The most reactive halogen is fluorine, and they become less reactive as you go down. Due to this, a more reactive halogen will displace a less reactive one. For example, when chlorine gas is bubbled through potassium bromide solution...
2KBr(aq) + Cl2 (g) ® 2KCl (aq) + Br2 (aq)
Chlorine and Chlorides
Chlorine has a very interesting reaction with water. It's a reversible reaction and also shows something called disproportionation [dis-pro-por-shon-ay-shon].
Disproportionation defines a reaction where one species (in this case chlorine) is simultaneously oxidized and reduced. If you have a look at chlorine in HCl, it has an [chemistry/physical/redox.html|oxidation number] of -1 and in hydrogen chlorate. This reaction has been used in water treatment to destroy bacteria, however this practice has been replaced by granular calcium hypochlorite Ca(ClO)2 which is less dangerous.
Another reaction we are going to look at is chlorine with cold, dilute sodium hydroxide (NaOH). This causes the following reaction to take place.
Cl2 + 2OH- ® Cl- + ClO- + H2O
This reaction is very important because it is used commercially to produce bleach!
Questions and Answers
I read that fluorine is the most reactive but I thought it was the least?
Small atom so electrons closer to nucleus- doesn't that mean it's less reactive??
- The reason Fluorine is the (most) reactive is that it's at the top of the Halogen group or 2nd to the right on the periodic table. The higher an element is, the more reactive it is.
- Outer shells that orbit the nucleus lack electrons which makes them enthusiastically grab electrons. Fluorine is a massively reactive element, is nonmetal and halogen, it's atomic weight is 18.9984032, it's atomic number is 9 and contains 10 neurons in the nucleus.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Group: VII: Halogens, Halides and Chlorine. (2017). In ScienceAid. Retrieved Apr 19, 2024, from https://scienceaid.net/chemistry/inorganic/halogens.html
MLA (Modern Language Association) "Group: VII: Halogens, Halides and Chlorine." ScienceAid, scienceaid.net/chemistry/inorganic/halogens.html Accessed 19 Apr 2024.
Chicago / Turabian ScienceAid.net. "Group: VII: Halogens, Halides and Chlorine." Accessed Apr 19, 2024. https://scienceaid.net/chemistry/inorganic/halogens.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Inorganic
Recent edits by: SmartyPants, Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)