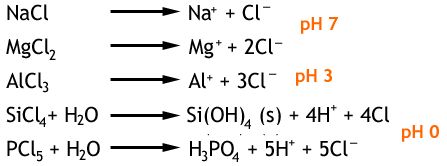Trends in Period 3 Elements
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Destiny, Jen Moreau and 1 other
Reactions of Period 3 Elements
Only three period 3 elements will react with water; sodium, magnesium and chlorine. Below are the reactions of sodium and magnesium with water. Sodium reacts quite vigorously with cold water. But for the reaction with magnesium to occur, steam must be used.
2Na (s) + 2H2O (l) ==>> 2NaOH (aq) + H2 (g) Mg (s) + H2O (g) ==>> MgO (s) + H2 (g)
Now we will look at the reactions of period 3 elements with oxygen. The reactions and some notes on them are in the table below.
All period 3 elements up to phosphorous will react with chlorine, these reactions and some notes on them are in the table below.
Acid-Base Properties of Oxides
Those oxides that are produced in the reactions above have various physical properties. Sodium and magnesium oxides have ionic structures and silicon, phosphorous and sulphur oxides have covalent bonding. Aluminium oxide on the other hand, has a type of boding that is somewhere in between. Since aluminium has a very high charge density it is able to distort the electron cloud so that aluminium oxide has a very covalent character.
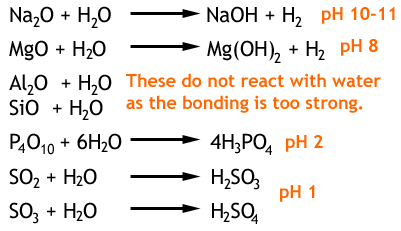
In the table below are the reactions of period 3 oxides with water, with the resulting pH.
You will notice that the trend in the pH of the solutions formed goes from alkaline to acidic. This is because the less electronegative sodium has a weak Na-O bond and the oxygen is more easily given up to react with H+. Further along though, a strong S-O bond keeps this together and more H+ is generated. The amphoteric aluminium oxide has a bonding which is both ionic and covalent in nature.
When these oxides are reacted with acids or bases, a neutralization occurs with a salt and water produced. Aluminium oxide is amphoteric, meaning it reacts with both acids and bases.
Reactions of Chlorides with Water
When period 3 elements are reacted with chlorine gas the chlorides X-Cl1-5 are produced in the order Na, Mg, Al, Si, and P. The sodium and magnesium chlorides are ionic structures which have high boiling points. Aluminium chloride will sublimate and the Si and P chlorides are both liquids at room temperature.
Below are the reactions of the period 3 chlorides with water, along with the pH values.
The covalent chlorides (aluminum, silicon, phosphorous) are hydrolysed by water and form acidic solutions that contain HCl. Whereas the ionic chlorides of sodium and magnesium simply dissolve.
Questions and Answers
Explain the trends in period 3 as it regards to solubility in water and ph value on the universal indicator?
Magnesium oxide,aluminium oxide,phosphorus pentoxide,glass and silica solubility in water. It does not explain fully why these compounds are not soluble in water and why the ph reading on the universal indicator varies for each compound. I have tried: .searching for appropriate explanation . trying different sites . looking for examples. I think it was caused by: Information is not in dept stuff not explained no theoretical based answers were provided
MgO and AlO are both soluble in water with low pH. Dissolution is a proton driven reaction here. Read this to understand more: Hydration of MgO(100) Surface Promoted at ⟨011⟩ Steps Akira Sasahara, Tatsuya Murakami, and Masahiko Tomitori The Journal of Physical Chemistry C 2015 119 (15), 8250-8257
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Trends in Period 3 Elements. (2017). In ScienceAid. Retrieved Apr 20, 2024, from https://scienceaid.net/chemistry/inorganic/period3.html
MLA (Modern Language Association) "Trends in Period 3 Elements." ScienceAid, scienceaid.net/chemistry/inorganic/period3.html Accessed 20 Apr 2024.
Chicago / Turabian ScienceAid.net. "Trends in Period 3 Elements." Accessed Apr 20, 2024. https://scienceaid.net/chemistry/inorganic/period3.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Inorganic
Recent edits by: Jen Moreau, Destiny, Taylor (ScienceAid Editor)