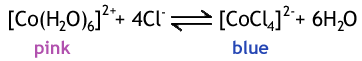Substitution Reactions
Edited by Jamie (ScienceAid Editor), Jen Moreau, vcdanht
Substitution Reactions
In the acidity reactions, an O-H bond is broken. However, the co-ordination bond between metal and ligand can be broken if a ligand substitution occurs.
Neutral Ligand Substitution
When ammonia is added to a solution it can produce the equilibrium:
NH3 + H2O ==>> NH4+ + OH-
This OH- will react with aqua ligands to form hydroxides, which is a precipitate. So when you add a little ammonia to a complex aqua ion, it will form a precipitate. If the ammonia is added in excess it will replace the water.
[M(H2O)6]2+ + 6NH3 ==>> [M(NH3)6]2+ + 6H2O
The above equilibrium actually occurs in individual steps where water is replaced one ligand at a time - so the coordination number stays the same. So there may be incomplete substitution as with hexaaquacopper where [Cu(NH3)4(H2O)2]2+ is formed.
Charged Ligand Substitution
Cl- ligands can be exchanged if concentrated hydrochloric acid is added to a solution, for example, the change with Co is...
Note that the co-ordination number changes from 6 to 4: from an octahedral to a tetrahedral complex. This is because the Cl- ion is large and the negative charge means the ligands repel each other.
Bidentate and Multidentate Ligand Substitution
Water ligands can also be replaced by bidentate and multidentate ligands. For example, the ligand ethane-1,2-diamine, which is simplified to en.
[M(H2O)6]2+ + en ==>> [M(H2O)4en] + 2H2O
Further substitution occurs and [M(en)3]2+ is formed. The equilibrium here is very much to the right, and the value of Kc is in the order of 1020. This is because the resulting complexes are much more stable.
This stability is a result of the chelate effect where chelate ligands make more stable compounds. Indeed, if metal ions are kept in excess EDTA4-, they said to be sequestered and the addition of OH- or carbonate ions - which normally forms a precipitate - will have no effect.
The reason for this is in the entropy change using the feasibility equation. In forming a chelate, �"S is large and positive, whilst the enthalpy change is very small so the �"G will always be very negative and the reaction highly feasible.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Substitution Reactions. (2017). In ScienceAid. Retrieved Apr 24, 2024, from https://scienceaid.net/chemistry/inorganic/substitution.html
MLA (Modern Language Association) "Substitution Reactions." ScienceAid, scienceaid.net/chemistry/inorganic/substitution.html Accessed 24 Apr 2024.
Chicago / Turabian ScienceAid.net. "Substitution Reactions." Accessed Apr 24, 2024. https://scienceaid.net/chemistry/inorganic/substitution.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Inorganic
Recent edits by: Jen Moreau, Jamie (ScienceAid Editor)