Alcohols
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Sim
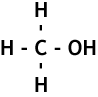
The alcohols are another homologous series of compounds that have the general formula CnH2n+1OH.
The Alcohol Series
The names of the alcohols follow the same pattern as the alkanes and the alkenes. Here are the first few, their names, chemical and structural formula:
| Methanol | Ethanol | Propanol | Butanol |
| CH3OH | C2H5OH | C3H7OH | C4H9OH |
Types of Alcohol
We have three different types of alcohol depending on the structure. They will react differently, which is why you need to know the differences, but they are very simple.
The easy way to work it out is the number of carbon atoms bonded to the COH group.
- 1A primary alcohol has 0 or 1 carbon on the COH.Primary.Advertisement
- 2The secondary alcohol has 2 carbons.Secondary.
- 3The tertiary alcohols have 3 carbon atoms bonded to the C-OH. Tertiary alcohols will not oxidize easily, so regarding the reaction, you won't see much happen.Tertiary.
Manufacturing Ethanol
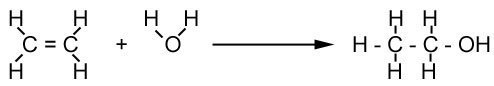
There are two methods of manufacturing ethanol - either from ethene or sugar.
Ethene
Producing alcohol from ethene is more common in developed countries like those in North America and Europe, where ethene is widely available. The following reaction between ethene and steam takes place with a phosphoric acid catalyst at 600°C and high pressure.
Sugar
The production of ethanol using sugar is more common in developing nations with less oil and ethene but a lot of agricultural produce. This method also makes use of material that would have otherwise gone to waste. Plant material is fermented for several days, and the following reaction takes place:
The ethanol produced is combustible, so can be used in car engines. This has become known as biofuel. Initially, it was believed to provide a more environmentally friendly source of fuel to replace fossil fuels. It appears, however, to do more harm than good by reducing the supply of food and encouraging deforestation to plant crops that can be fermented.
Uses of Ethanol
Ethanol is by far the most commonly used alcohol, including the alcohol in your cocktails. It has numerous applications, including:
- As a solvent for paints and perfumes. When the product is applied, the ethanol evaporates.
- It is used as fuel. Most notably in Brazil, where either pure ethanol or a mix with petrol (gas) is used to fuel the majority of cars. This can be a carbon neutral way to fuel transport, because although carbon dioxide is produced in combustion, manufacturing it by fermentation means all CO2 is absorbed by growing the plants to ferment.
For making other compounds like ethanoic acid (see below) or esters.
Oxidation
Alcohols can be oxidized by using chemicals like acidified potassium dichromate in warm conditions. The products vary depending on the type of alcohol. For example - a primary alcohol will oxidize to to an aldehyde and given further oxidation it will become a carboxylic acid. Have a look at the examples below using ethanol.
The [O] is notation used to represent oxidation. Ethanol first becomes the aldehyde ethanal and produces water. With further oxidation (step 2) this is oxidized to ethanoic acid.
A secondary alcohol can will be oxidized to a ketone with the functional group CO. The diagram below shows what the ketone propanone looks like.
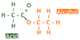
Ethanoic Acid
Formerly known as acetic acid, ethanoic acid a type of carboxylic acid with the molecular formula of C2H4O2 and structural formula of:
It is the acid found in vinegar, which is why if wine is left, the ethanol in it oxidizes to become ethanoic acid:
Questions and Answers
Yes sir, List three uses of trioxonitrate acid and alcohol?
List three uses of trioxonitrate acid and alcohol in details
The process where trioxonitrate (or nitric acid) reacts with alcohol is called nital. This process is mainly used for etching of metals and showing the microstructure of carbon steels.
Hi is proponal a primary secondary, I'm thinking secondary because I see 2 C bound to the COH?
Hi is propanal a primary secondary, I'm thinking secondary because I see 2 C bound to the COH
ScienceAid QnA. This section is not written yet. Want to join in? Click EDIT to write this answer.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Alcohols. (2017). In ScienceAid. Retrieved Apr 20, 2024, from https://scienceaid.net/chemistry/organic/alcohols.html
MLA (Modern Language Association) "Alcohols." ScienceAid, scienceaid.net/chemistry/organic/alcohols.html Accessed 20 Apr 2024.
Chicago / Turabian ScienceAid.net. "Alcohols." Accessed Apr 20, 2024. https://scienceaid.net/chemistry/organic/alcohols.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Organic
Recent edits by: Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)