Alkanes
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
About Alkanes
Alkanes are saturated hydrocarbons, since they only have single covalent bonds. Examples of Alkanes, are the gases produced by fractional distillation.
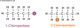
Alkanes have the general formula of CnH2n+2, below is a table of the first four alkanes in increasing order of carbon atoms.
| Name | Formula | Structure |
|---|---|---|
| Methane | CH4 | |
| Ethane | C2H6 | |
| Propane | C3H8 | |
| Butane | C4H10 |
Combustion
These products make very good fuels as their combustion is exothermic. Here are two ways combustion takes place, depending on how much oxygen there is.
Complete Combustion
Complete combustion takes place when there is a plentiful supply of oxygen. Its products are carbon dioxide and water; see the example below. C4H10 + 6½O2 ==>> 4CO2 + 5H2O.
Incomplete Combustion
'Incomplete combustion takes place when only a limited supply of oxygen is available. Its products are water, and carbon or carbon monoxide water, an example of the reaction is.
CH4 + O2 ==>> C + 2H2O
CH4 + 1½O2 ==>> CO + 2H2O
However, if you think about this as happening in your car engine, then because of impurities in the fuel, other chemicals are produced, notably nitrous oxides and sulphur dioxide, which is responsible for acid rain. Fortunately, we have a way of removing the more harmful chemicals - the use of a catalytic converter. The catalytic converter removes carbon monoxide, nitrogen oxide and hydrocarbons from the exhaust. They contain a thin layer of metals such as platinum and rhodium that act as a catalyst to convert the harmful gases into less harmful ones.
Chlorination
This is the process where chloromethane (methyl chloride) is produced from chlorine and methane. If you did this experiment outside at night, nothing would happen; but if you perform the experiment in a sun bed, it will react explosively. This is because UV light provides the energy to break the Cl - Cl bonds.
The overall equation of this reaction is as above, however there are three stages to it, and at each one, we shall study the mechanism.
- 1The first stage is initiation, where the UV light breaks the bond in the Chlorine Molecule to form two chlorine free radicals. This means it has an unpaired electron and is therefore very reactive.Initiation.Advertisement
- 2
- 3The final step is termination. This is when two free radicals combine. Mostly this means the Cl and CH3 because these collisions are more probable. However, it is also possible for two CH3 to join and make ethane.Termination.
Know the Difference
You can tell the difference between an alkane and alkene by adding bromine water to the substance and giving the tube a shake.
- When bromine water is added to an alkane, the solution will turn red-brown (the color of bromine water) and stay that way while shaking.
- Alkenes however will turn red-brown, but then the color disappears while shaking. This is because the bromine has reacted with the alkene.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Alkanes. (2017). In ScienceAid. Retrieved Apr 23, 2024, from https://scienceaid.net/chemistry/organic/alkanes.html
MLA (Modern Language Association) "Alkanes." ScienceAid, scienceaid.net/chemistry/organic/alkanes.html Accessed 23 Apr 2024.
Chicago / Turabian ScienceAid.net. "Alkanes." Accessed Apr 23, 2024. https://scienceaid.net/chemistry/organic/alkanes.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Organic
Recent edits by: Jamie (ScienceAid Editor)