Esters
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Jen Moreau, vcdanht and 2 others
Esters are another homologous series of organic compounds. They are sweet-smelling and have a fairly complicated structure for this level of|organic chemistry. When ethanol and formic acid react (alcohol and caboxylic acid), ethyl formate is formed.
Manufacture
An ester is the result of the reaction between a carboxylic acid and alcohol.
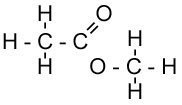
Ethyl ethanoate is produced by the reaction of ethanoic acid (the acid) and ethanol (the alcohol).
Ethanoic Acid + Ethanol ==>> Ethyl Ethanoate + Water CH3COOH + C2H5OH ==>> CH3COOC2H5 + H2O
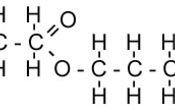
It has the following structure. Note that you can recognize a part resembling ethanoic acid and another ethanol.
Naming esters can be quite difficult, however, once you have drawn out the structure it should be easy. The part that is acid ends in -oate (methanoate, ethanoate) and the part that is alcohol is the ethyl group (methyl, ethyl, pentyl etc).
Draw out the structure or better still, use a model set to learn how to draw any ester in an exam.
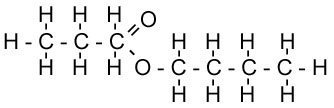
| Methyl Ethanoate | Butyl Propanoate |
|---|---|
| Ethanoic Acid + Methanol | Propanoic Acid + Butanol |
The combinations are numerous, but hopefully, you can see how the pattern works. Using the rules of naming (see above) and the basic rules of organic chemistry nomenclature, you should find esters very easy.
Pear Drops
Esters are sweet-smelling liquids used to flavor foods and sweets. Ethyl ethanoate, for example, is used in pear drops, (a hard candy) because of its distinctive aroma which is similar to pears. And different esters are used as flavorings from honey (methyl phenyl ethanoate) to parsnip (octyl butyrate) to rum (propyl isobutyrate). Esters are responsible for the taste of raspberries.
Questions and Answers
Where does the extra hydrogen go when formic acid and ethanol are synthesized to create ethyl formate?
The hydrogen at the formic acid is removed, and "replaced" by two carbons and four hydrogens from the ethanol. Then the hydroxyl group from the ethanol and the hydrogen that came off of the formic acid create water (dehydration synthesis). That leaves one hydrogen from ethanol, this will be released by the reaction as hydrogen gas.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Esters. (2017). In ScienceAid. Retrieved Apr 19, 2024, from https://scienceaid.net/chemistry/organic/esters.html
MLA (Modern Language Association) "Esters." ScienceAid, scienceaid.net/chemistry/organic/esters.html Accessed 19 Apr 2024.
Chicago / Turabian ScienceAid.net. "Esters." Accessed Apr 19, 2024. https://scienceaid.net/chemistry/organic/esters.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Organic
Recent edits by: SarMal, vcdanht, Jen Moreau