Haloalkanes
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Jen Moreau, SmartyPants
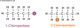
Nucleophilic Substitution
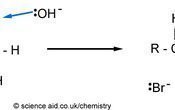
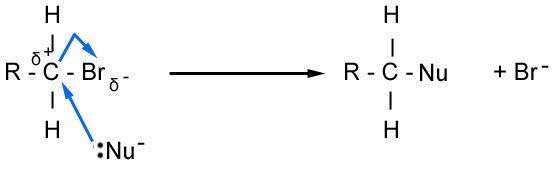
Haloalkanes contain a polar bond on the functional group. Because the halogen is electronegative, it becomes δ- and the carbon is positive. This means it is susceptible to nucleophilic attack by ions. The strength of the C - Halogen bond also influences the rate of substitution. The C-F bond requires a quite a bit (484 kJmol-1) of energy to break, however, the C-Br bond is easier to break so the rate of reaction is faster. The mechanism below uses the notation :Nu- to refer to the nucleophile, which can either be OH- or NC-.
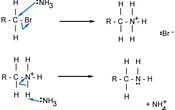
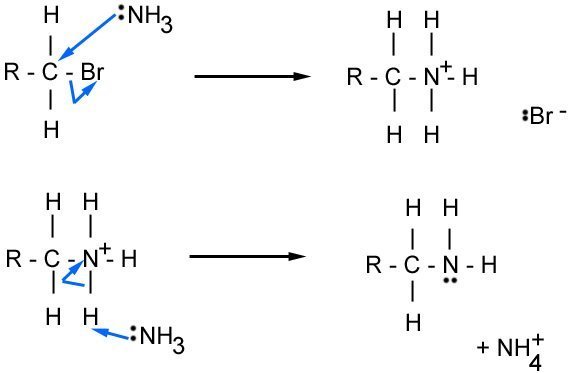
The mechanism below shows what happens with ammonia and a haloalkane
The bromine ion and NH4 join to make NH4Br, and the final product is an amine
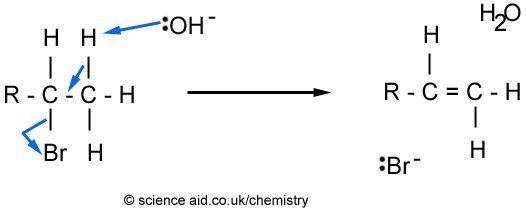
Elimination
In the first mechanism, the OH ion replaces the Br and forms an alcohol. However, the OH- can also act as a base, where an elimination reaction occurs, as outlined below.
How do you decide whether substitution or elimination is going to happen? There are several factors that will determine what will happen. Have a look at the table below.
| Haloalkane Structure | Primary haloalkanes prefer substitution.
Secondary haloalkanes will do both at the same rate. Tertiary haloalkanes prefer elimination. |
| Base Strength | Elimination is more likely as the strength of the base increases. |
| Temperature | Higher reaction temperatures make elimination happen more. |
If you are not sure about the differences between primary, secondary and tertiary then look at the page alcohols, as the same rules apply.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Haloalkanes. (2017). In ScienceAid. Retrieved Apr 25, 2024, from https://scienceaid.net/chemistry/organic/haloalkanes.html
MLA (Modern Language Association) "Haloalkanes." ScienceAid, scienceaid.net/chemistry/organic/haloalkanes.html Accessed 25 Apr 2024.
Chicago / Turabian ScienceAid.net. "Haloalkanes." Accessed Apr 25, 2024. https://scienceaid.net/chemistry/organic/haloalkanes.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Organic
Recent edits by: Jen Moreau, Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)