Further Enthalpy: Enthalpies, Born-Haber Cycle, Mean Bond Enthalpy
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
The Enthalpies
Previously, the enthalpies of formation and combustion have been discussed, however, there are numerous others. In the table below, some of these have been outlined, with a definition given.
| Enthalpy | Definition |
|---|---|
| Atomisation Hθa | The standard enthalpy change when 1mol of gaseous atoms are formed. Depending on the standard state of the element, this could also be called the enthalpy of sublimation if going from solid to gas. |
| Bond dissociation, Hθdiss | The standard enthalpy change when 1mol of gaseous covalently bonded molecule is broken to form 2 radicals. So the enthalpy change for A (g) ==>> X. (g) + Y. |
| Electron Affinity, Hθea | The standard molar enthalpy change when an electron is added to an atom in the gas phase. For example: Cl (g) + e- ==>> Cl- (g) |
| Lattice Enthalpy, HθL | The standard enthalpy change accompanying dissociation: the separation of 1 mol of solid ionic lattice into gaseous ions; formation the creation of 1 mol of solid from gas ions. Note that formation enthalpies are always negative and dissociation enthalpies are always positive. |
| Hydration, Hθhyd | The standard molar enthalpy change for the process: X+/- (g) ==>> X+/- (aq). |
| Solution, Hθsol | The standard enthalpy change when 1mole of ionic solid dissolves in enough water that none of the dissolved ions interact with each other. |
Born-Haber Cycle
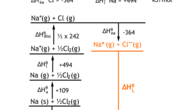
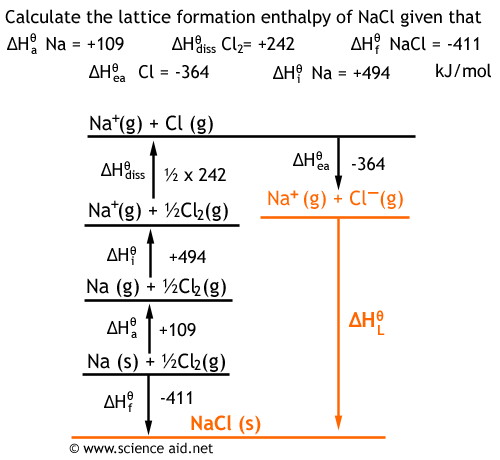
Born-Haber cycles expand Hess's Law by breaking up the lattice formation enthalpy into numerous other steps. Given the data for various enthalpies, it shouldn't be too difficult to deduce various reactions. Below is an example.
The enthalpy we are finding has been highlighted. It is the lattice enthalpy of formation of NaCl. It has the reaction:
Na+ (g) + Cl- (g) ® NaCl (s)
Once the cycle has been constructed, calculating the enthalpy is a simple case of following the alternative route for the reaction. Remember that if you go in the opposite direction of the arrow, the sign of the enthalpy is reversed (i.e.. +20 become -20 or vice versa). 364 - (½x242) - 494 - 109 - 411 711 kJ mol-1
It is possible to calculate the enthalpy of any reaction using the same principle of making several steps of different enthalpies, for example, the enthalpy of solution. Be careful of state symbols and the number of moles involved though!
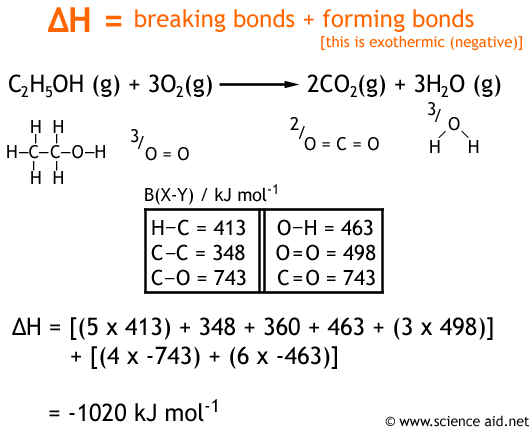
Mean Bond Enthalpy
There is a certain amount of energy required for breaking bonds, and energy released in forming bonds. Therefore, it should be a simple case of using the amount of energy in each bond to calculate the enthalpy change of a reaction. However, this is not possible, because the bond enthalpy for the same bond will vary in different compounds and conditions. In the absence of sufficient enthalpies, an average of the energy in each bond can be used as an estimate. This is called the mean bond enthalpy. The example below shows how they can be used.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Further Enthalpy: Enthalpies, Born-Haber Cycle, Mean Bond Enthalpy. (2017). In ScienceAid. Retrieved Apr 16, 2024, from https://scienceaid.net/chemistry/physical/moreenthalpy.html
MLA (Modern Language Association) "Further Enthalpy: Enthalpies, Born-Haber Cycle, Mean Bond Enthalpy." ScienceAid, scienceaid.net/chemistry/physical/moreenthalpy.html Accessed 16 Apr 2024.
Chicago / Turabian ScienceAid.net. "Further Enthalpy: Enthalpies, Born-Haber Cycle, Mean Bond Enthalpy." Accessed Apr 16, 2024. https://scienceaid.net/chemistry/physical/moreenthalpy.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physical
Recent edits by: Jamie (ScienceAid Editor)