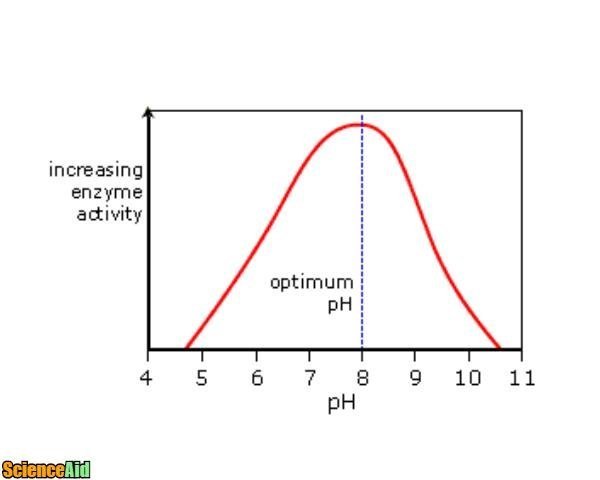
Enzymatic Activity and pH Buffers
Edited by Chameleon, Jen Moreau, SarMal
Metabolism of all biological molecules, protein synthesis, protein activation, lipid remodelling, lipid synthesis, and other biological processes all rely on enzymatic activity. There are many factors that can affect enzymatic activity such as including pH. pH refers to the concentration of hydrogen ions in a system. The concentration of hydrogen ions influences the enzymatic activity by adding or removing hydrogen ions to the enzymes. As hydrogen is positively charges, it will bind to the negative charge on enzymes. Buffers are solutions that maintain the concentration of hydrogen ions in a system or a solution. There are endogenous proteins that balance the hydrogen ion concentration inside a cell and within the cell environment; these proteins are called proton pumps. However, in vitro experiments it is necessary to have pH buffers in the system where enzymatic activity occurs because of the absence of these proteins.
Buffers
A mixture of an acid and its conjugate base will have the same concentration of hydrogen ion in spite of the reaction that occurs. This only occurs when the buffer is made of a strong acid and a strong base, or a weak acid and a weak base. If either the acid or the base is stronger or weaker than its conjugate, the pH will change very slightly. The best pH buffers are the strong acid-strong base buffers or the weak acid-weak base buffers. [1]
The addition of various compounds to a solution can decrease or increase its hydrogen ion concentration. Buffered solutions will maintain the hydrogen ion concentration regardless of the addition of other chemical compounds.
pH requirements of some major classes of enzymes
Oxidoreductases
These are enzymes that catalyze the transfer of electrons to and from molecules. They work with pH 7.8 - 8.0, which is a slightly alkaline condition. The buffer that can maintain this pH is Tris buffer. Tris (hydroxymethyl) aminomethane is a base; it can be used in conjunction with Hydrochloric acid to make a buffer for the desired pH. Tris buffer is widely used in experimental procedures; however, this does not imply that it is the best buffer. It can have undesirable effects on biological systems, it is temperature sensitive, and can be active as a primary amine compound. In spite of these cons, it has worked for many experiments and is still used till date. [2]
Transferases
This is a wide class of enzymes that transfer various functional groups from one molecule to another. The pH requirement for this class of enzymes would vary; however, an example of pH requirement is pH 6.5 - 7.5. This is a requirement for UTP-monosaccharide-1-phosphate uridylyltransferase. This enzyme can work in a slightly acidic to a neutral environment. Buffers that can maintain this pH include phosphate buffers, BES (N, N-Bis-(2-hydroxyethyl)-2-aminoethanesulphonic acid) buffers, and MOPS (3-(N-morpholino) propane sulphonic acid) buffers. Phosphate buffers are commonly used because they are easily obtained and prepared. [3]
Hydrolases
These enzymes cleave bonds between molecules by hydrolysis. Succinylcholine-Diaminopimelate desuccinylase works at a pH of 8.0, which is slightly alkaline. Barbital buffers, Tris buffers, and Phosphate buffers can maintain a solution at this pH.
Lyases
These enzymes are similar to hydrolases. They cleave bonds and remove chemical groups, but not by hydrolysis. A characteristic of their catalysis is that they leave a double bond in one the molecules. Aspartate ammonia-lyase is an example of this class. It works with a pH range of 7-8.5. Again, Tris buffers, Phosphate buffers, and Barbital buffers are suitable for maintaining the pH within this range.
Isomerases
These are enzymes that transfer chemical groups from one molecule to another, and consequently, form an isomer of the molecules involved. Glucose isomerase work within a pH range of 7- 8.5. Tris buffers, Phosphate buffers, and Barbital buffers are suitable for maintaining the pH within this range. Phosphate buffers are more commonly used for glucose isomerase reactions.[4]
Ligases
These are enzymes that catalyze the formation of new bonds by condensing and hydrolyzing high energy molecules. An example of this class of enzymes is glutamine synthetase. The pH requirement for this enzyme is pH 7 - 8. Tris buffers and Phosphate buffers are suitable for maintaining the pH within this range.
Most enzymes react optimally in slightly acidic to slightly alkaline environments. The pH requirements for enzymes vary according to their class, activity, environment, and isotype.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Enzymatic Activity and pH Buffers. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/Enzymatic_Activity_and_pH_Buffers
MLA (Modern Language Association) "Enzymatic Activity and pH Buffers." ScienceAid, scienceaid.net/Enzymatic_Activity_and_pH_Buffers Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Enzymatic Activity and pH Buffers." Accessed Apr 27, 2024. https://scienceaid.net/Enzymatic_Activity_and_pH_Buffers.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
- ↑ Beynon R, Easterby J. Buffer solutions. 1st ed. Oxford: IRL Press; 1996.
- ↑ Perrin D, Dempsey B. Buffers for pH and metal ion control. 1st ed. Kluwer Academic Publishers; 1974.
- ↑ Gajera H, Patel S, Golakiya B. Fundamentals of biochemistry. 1st ed. Lucknow: IBDC; 2008.
- ↑ Buffer tables. Microscopy.berkeley.edu. 2017. Available at: http://microscopy.berkeley.edu/Resources/instruction/buffers.html [Accessed April 5, 2017].
Article Info
Categories : Biology
Recent edits by: Jen Moreau, Chameleon