Electron Arrangement
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Jen Moreau, Sharingknowledge and 1 other
About Electron Arrangement
This topic often confuses people so don't worry if you don't understand it the first time. Read up on electron arrangement in textbooks and get other people to explain it to you. Before this level of study, you will have been told that electrons have fields of 2,8,8 maximum capacity. Well, this is not true. As well as main energy levels, there are also sub-shells. The table below outlines this information.
| Main Level | Sub-level | Electron capacity |
|---|---|---|
| 1 | s | 2 |
| 2 | s | 2 |
| p | 6 | |
| 3 | s | 2 |
| p | 6 | |
| d | 10 |
For these sub-shells, it's important to note that the 4S requires less energy for electrons to enter it, then the 3d. Other than this, the electrons fill up each successive sub shell in order to form electrons. Below are the electron configurations of some elements.
Highlighted on the image are two features:
- 1At Scandium, the electrons in 3d start to fill up after 4S.Scandium (Sc).Advertisement
- 2The second is [Ne], this is a shorthand way of writing the electronic configuration. the bit highlighted means the electron configuration of Neon than the rest. You can use any noble gas, dependent on its size.Electronic Configuration [Ne].
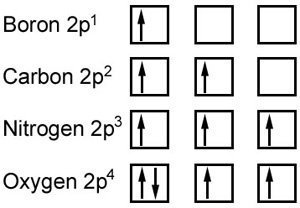
Hund's Maximum Multiplicity Rule
This rule is an important one when considering electron arrangement and ionization energy. It states that in subshells, the electrons will as fill singly as much a possible, before doing so in pairs. This is to be as energy efficient as possible. It is demonstrated below using spin diagrams.
Ionization Energy
The first ionization energy is defined as: The energy/enthalpy change when one mole of electrons is removed from one mole of a gaseous element, the equations for the first and second ionization energies are shown below.
The ionization energies of elements have a distinctive pattern, which provides evidence for the structure of electrons explained above.
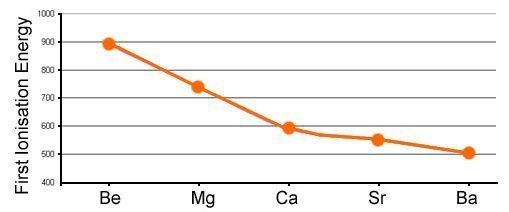
Group II patterns
Down the groups, there is a decrease in first ionization energy. This is because of the following factors:
- 1This is the distance of the outermost electron to the nucleus. As you go down the groups, this distance gets bigger, so it becomes easier to remove electrons (less energy is required) as they are further from the attractive force of the nucleus.Atomic Radius.
- 2As you go down the group there is a new n (main) electron level. This means that other electrons are below the outermost. Because of this, there is a degree of repulsion, which means the electron is more readily lost.Electron Shielding.
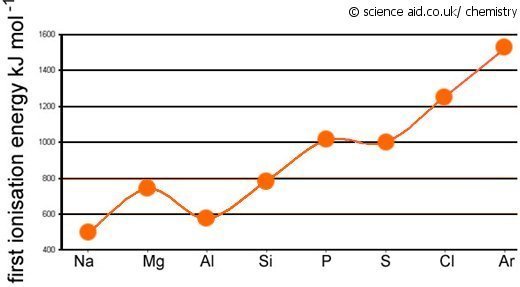
Period III Patterns
The pattern of first ionization energies across a period is a little more complex because it doesn't show a perfect pattern. However, there is a general increase along the period because of increasing nuclear charge. The force of attraction from the increasing number of protons means the electrons are being held in place with more force so more energy is required to remove them.
There is a drop in first ionization energy from magnesium to aluminum. This is because from Al, the electrons start filling the 3p subshell, and because this is in a higher energy level, it is lower.
The next slight fall is from Phosphorus to Sulphur. This is because of Hund's maximum multiplicity as explained above. The electrons begin to pair up, and so less energy is required to remove it since the two electrons in a pair repel each other slightly.
Questions and Answers
How do I explain the first and second ionization energy of magnesium?
The first ionization energy of magnesium is larger than the first ionization of other sulfates because magnesium has an additional proton in its nucleus. When comparing with sodium, for example, the first ionization energy of magnesium is larger than sodium because of the additional proton. This additional proton in the nucleus is necessary to keep electrons in the 3s orbit:
The second ionization energy of magnesium is much larger than the first. This is because it takes much more energy to take an electron from an ion that is positively charged than from an atom that is neutral. Logically, the third ionization energy of magnesium is even larger.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Electron Arrangement. (2017). In ScienceAid. Retrieved Apr 29, 2024, from https://scienceaid.net/chemistry/fundamental/electrons.html
MLA (Modern Language Association) "Electron Arrangement." ScienceAid, scienceaid.net/chemistry/fundamental/electrons.html Accessed 29 Apr 2024.
Chicago / Turabian ScienceAid.net. "Electron Arrangement." Accessed Apr 29, 2024. https://scienceaid.net/chemistry/fundamental/electrons.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Fundamental
Recent edits by: Sharingknowledge, Jen Moreau, Taylor (ScienceAid Editor)