Fuel Cell
Edited by Simo, Jen Moreau, SarMal, Sharingknowledge
Fuel cells are the energy sources of the future technological advances for all sorts of gadgets, including cars, laptops and even airplanes. The fuel cell is a device that uses a chemical reaction to create electricity directly from the fuel (Hydrogen, natural gas). This electricity is created with a higher efficiency compared to other thermal engines. A standard fuel cell can have an 80% efficiency; meanwhile, a good thermal engine is only about 30% efficient.
The traditional example of a fuel cell is a hydrogen fuel cell. A hydrogen fuel cell uses hydrogen and oxygen reaction to release energy. The chemical equation for such reaction is given by:
The energy can be released in the form of an uncontrolled explosion, but luckily there are more controlled ways to release the energy using a fuel cell. In the fuel cell, it is possible to break the chemical reaction into two halves by using a membrane to keep the two gasses (hydrogen, oxygen) apart. On one side, called the anode, the hydrogen molecules lose electrons. e^- and become protons H^+. The protons will then diffuse through the electrolyte in the center of the fuel cell. On the other side, called the cathode, those protons will react with oxygen. O_2 to form water H_2 O. For this to happen electrons on the anode side need to flow through an external circuit which produces ELECTRICITY.
Different types of fuel cells use different electrolytes, different operating temperature, and different fuels.
Fuel Cell Characteristics
The relation between the fuel cell current and fuel cell voltage is expressed using the equation:
V_fc=E_0 - V_ac t- V_ohm - V_conc
Where
V_fc : the fuel cell voltage.
E_0: ideal fuel cell voltage.
V_act : Activation polarisation.
V_ohm: Ohmic polarization.
V_conc : Concentration polarisation.
Activation Polarization
The activation losses are due to the start of chemical reactions at the anode and the cathode. Since the oxidation reaction of hydrogen is faster than that of reduction of oxygen, the losses are primarily due to the cathodic losses. The equation of this loss can be expressed using Tafel's equation:
V_act=A ln((I_fc+i_n)/i_0 )
Where
A: Tafel's slope.
I_fc: fuel cell current.
I_n: Leakage current inside the membrane of the fuel cell.
I_0: current at zero loads.
The Ohmic Polarization
The PEM cell possesses an ohmic behavior modeling opposition of the electrodes and the bipolar plates to the circulation of the electrons, in addition to the electrolyte resistance to the passage of the protons.
V_ohm=R_m (I_FC+i_n )
R_m: the total electric resistance of the fuel cell.
Concentration Polarization
The voltage drop due to the loss of concentration is manifested at high current density, is explained by the gas consumption which decreases the partial pressures. It is linked to the current delivered by the fuel cell and to the limiting current for which all the fuel (hydrogen) is consumed. This relationship is expressed by:
V_conc=-Bln (1-(I_fc+i_n)/i_L )
B: Mass transfer constant.
I_L: Limit current for which all the fuel is consumed.
Fuel Cell Characteristic Plot
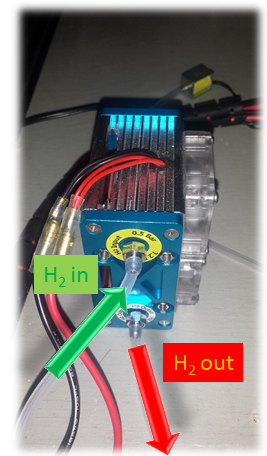
Example. A small prototype of 30 W fuel cell used to supply small applications such as drones or an electric robot.
The electrical circuit is connected to the black and red cables of the fuel cell, meanwhile, hydrogen supply is connected through the green and red arrows. This kind of fuel cells operates on the atmospheric pressure, which means it will just take the oxygen from the air for its chemical reactions.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Fuel Cell. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/Fuel_Cell
MLA (Modern Language Association) "Fuel Cell." ScienceAid, scienceaid.net/Fuel_Cell Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Fuel Cell." Accessed Apr 27, 2024. https://scienceaid.net/Fuel_Cell.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Engineering
Recent edits by: SarMal, Jen Moreau, Simo