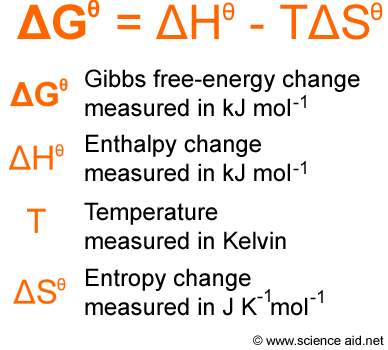Entropy
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), vcdanht
The Definition and Importance of Entropy
- 1Spontaneous change is a change that occurs naturally, without external intervention. In relation to chemical reactions, those reactions that occur naturally will often release energy, and so are exothermic. A chemical reaction that releases energy by heat or light is an exothermic reaction. Expressed in a chemical equation: reactants → products + energy.Exothermic Process.Advertisement
- 2This is when the energy is absorbed into the product - the opposite of exothermic. Usually, the absorption is achieved by heat. Some spontaneous reactions are endothermic, so there must be another factor we need to consider - entropy.Endothermic Process.
- 3This is the degree of disorder there is in a system. There is no energy available to complete the work. The more disorder there is in a system, the higher the value of entropy will be (S). The disorder of a system is very closely linked to the phase it is in.Entropy.
If you take the above example:
- A jar of bromine is next to an empty jar.
- When the divider between the two of them is removed, the bromine will fill both jars evenly.
- Why doesn't it fill the other entirely?
- Because there are many more probabilities of there being an even distribution than not, i.e.. it will spontaneously diffuse evenly.
Calculating Entropy Change
Entropy is affected by temperature. This is shown in the diagram below. Entropy increases gradually with the temperature, but then there is a sudden jump when the phase changes. The biggest jump occurs when it changes from a liquid to a gas. This is because there are more ways of arranging a gas than a liquid. Ions and molecules in solution also have higher entropy.
Calculating entropy change simply requires you to use given entropy values for particular substances, and use the following equation. They have the units J K-1 mol-1. It is possible to have absolute entropy since at 0 Kelvin, there is no entropy.
Feasibility of Reactions
As discussed above, exothermic reactions occur quite easily, and reactions that are endothermic but result in an increase in disorder, are also likely to happen. Therefore, it is possible to calculate the feasibility of a reaction by calculating the Gibbs free-energy change (ΔG).
A reaction is said to be feasible if ΔG is negative. For example: Determine whether the following reaction is feasible at 298K given that it has an enthalpy change of -825 kJ mol-1 and an entropy change of -272 J K-1 mol-1.
2Fe (s) + 1.5O2 (g) ® Fe2O3 (s)
ΔG = ΔH - TΔS ΔG = -825 - (298 x -272)/1000 ΔG = -744 kJ mol-1
Since ΔG is negative, the reaction is feasible, and the reaction will occur. Notice also how it was necessary to divide the ΔS by 1000 to convert it from J to kJ, alternatively you could multiply ΔH by 1000 to convert this from kJ to J.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Entropy. (2017). In ScienceAid. Retrieved Apr 28, 2024, from https://scienceaid.net/chemistry/physical/entropy.html
MLA (Modern Language Association) "Entropy." ScienceAid, scienceaid.net/chemistry/physical/entropy.html Accessed 28 Apr 2024.
Chicago / Turabian ScienceAid.net. "Entropy." Accessed Apr 28, 2024. https://scienceaid.net/chemistry/physical/entropy.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physical
Recent edits by: Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)