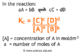Hess's Law
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
Introduction to Hess's Law
Hess's law is based on the first law of thermodynamics which says that energy can not be created or destroyed but can be converted from one form to another. Mr Hess used this principle and related it to chemical reactions, creating, Hess's Law:
Hess's Law says that the enthalpy change if a reaction depends only on the initial and final states of the reaction and is independent of the route by which the reaction may occur. In practical terms this means that you can do other reactions to get the energy for the main one.
Using Cycles
If you regularly commute somewhere by car, you won't have just one route. What if one of the roads is closed? Likewise, there are several different ways you could go, and this very principle can be used in Chemistry since Mr. Hess said it doesn't matter which way we go - the energy will be the same.
- 1
- 2We can also do a similar thing with formation. This is defined as the creation of a molecule from the elements that make it up in their standard states. Therefore this means H2 is used in the formation, not H. The equation for the formation of ethanoic acid:Formulation.
But remember that the enthalpy of formation for an element is 0, because it is already in its standard state.
Bond Enthalpies
The mean bond enthalpy is the amount of energy required to break a particular bond. For example, in methane there are 4 C-H bonds. The two following reactions show these being broken:
So the mean bond enthalpy for the C-H bond is 1664/4 = 416.
You can now use these bond enthalpies to calculate the energy in the bonds of all molecules.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Hess's Law. (2017). In ScienceAid. Retrieved Apr 26, 2024, from https://scienceaid.net/chemistry/physical/hess.html
MLA (Modern Language Association) "Hess's Law." ScienceAid, scienceaid.net/chemistry/physical/hess.html Accessed 26 Apr 2024.
Chicago / Turabian ScienceAid.net. "Hess's Law." Accessed Apr 26, 2024. https://scienceaid.net/chemistry/physical/hess.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physical
Recent edits by: Jamie (ScienceAid Editor)