Carbohydrates
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
About Carbohydrates
Carbohydrates are molecules that contain carbon, hydrogen and oxygen (and sometimes sulphur and nitrogen). They are extremely important for life since they are used for storing and transporting energy. To learn how to test for them, see tests.
Monosaccharides
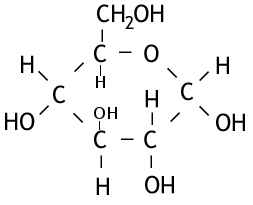
These are the simplest form of carbohydrates, being made of only one sugar; and often have a sweet taste. An example of a monosaccharide you may have heard of is glucose. It has the formula C6H12O6 and the following structure:
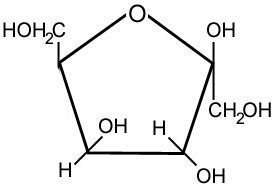
Glucose consists of a ring of 5 carbons and an oxygen atom around which is a series of H, OH and a CH2OH. The structure can be simplified to a polygon where each point represents a carbon. Fructose has the same formula as glucose, but a different structure, consisting of a 4 carbon and 1 oxygen ring. For this reason it is said to be an isomer of glucose, and has the following structure.
Disaccharide
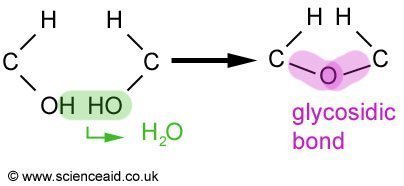
A disaccharide is made up of two monosaccharides and has the general formula Cn(H2O)n-1. Examples of them include maltose and sucrose. But before we look at them, let's take a look at how disaccharides are formed.
In the condensation reaction, an OH from one of the monosaccharides and H from the other are removed and produce water. Leaving an oxygen atom that acts as the bond between the two molecules. This is called a glycosidic bond. The breaking up of a disaccharide is a hydrolysis reaction and involves the addition of water. Maltose and sucrose are composed as follows.
Maltose = Glucose + Glucose Sucrose = Glucose + Fructose
Polysaccharide
A polysaccharide is a carbohydrate polymer where the monomers (single units) are monosaccharides. The monomers are joined by glycosidic links. They tend to be insoluble (do not dissolve) and have a very dull taste. Examples of polysaccharides are starch and glycogen, both made of glucose but arranged slightly differently. The picture below is a simple representation to help you understand the structure of a polysaccharide. So you can imagine, for example, that each green circle is a glucose, all joined together in a great long chain to make starch
Uses
As mentioned in the introduction, carbohydrates are extremely important in life. The table below outlines just a few examples of the fundamental uses of carbohydrates.
| Use | Type of Carbohydrate | Example |
|---|---|---|
| Respiration | Monosaccharide | Glucose |
| Component in RNA and DNA | Monosaccharide | Ribose and Deoxyribose |
| Building cell walls | Polysaccharide | Cellulose or Murecin |
| Energy storage | Polysaccharide | Starch or glycogen |
| Sweeting your food (table sugar) | Disaccharide | Sucrose |
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Carbohydrates. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/biology/biochemistry/carbohydrates.html
MLA (Modern Language Association) "Carbohydrates." ScienceAid, scienceaid.net/biology/biochemistry/carbohydrates.html Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Carbohydrates." Accessed Apr 27, 2024. https://scienceaid.net/biology/biochemistry/carbohydrates.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Biochemistry
Recent edits by: Jamie (ScienceAid Editor)