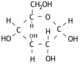Lipids
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
Lipids are fats and oils. They are important to living things because they form membranes. They are made of carbon, hydrogen and oxygen.
Triglycerides
Below is a diagram of a glycerol molecule and then a fatty acid. These are two important parts to creating a triglyceride.
The Glycerol
The glycerol is a simple molecule.
Fatty Acids
Fatty acids consists of a methyl group, a hydrocarbon chain which can be from 14 to 22 carbon atoms long (but always an even number), and it ends in a carboxyl group. Yet, the methyl group can be considered part of the hydrocarbon chain, and known collectively as the R group. Therefore the fatty acid can be written like this: RCOO-. The carboxyl end of the fatty acid is polar and so it is hydrophilic (it likes water)
The fatty acid with only single bonds between the carbon molecules is said to be saturated because they are saturated with hydrogen, however, if there are double bonds between carbon molecules (C = C = C) all of its bonds are used up and no hydrogens can get in. This means in is unsaturated.
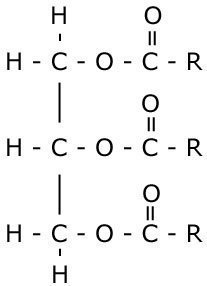
Creating a Triglyceride
And now, we will create a triglyceride. This is simply - one glycerol plus three fatty acids which makes a structure that looks a bit like this:
It is an example of a condensation reaction where 3 water molecules are lost for every one molecule of triglyceride produced.
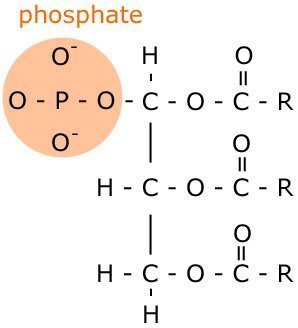
Phospholipids
A phospholipid is similar in structure to the triglyceride, but instead of one of the fatty acid chains, it has a phosphate.
As you can see, the phosphate part is polar, and so is hydrophilic. When it comes into contact with water, the 'tails' of the phospholipid face each other, with the 'heads' facing outwards. This structure is called a micelle
Alternatively, the phospholipids might form a phospholipid bilayer (similar to that seen in cell membranes). This is a ring of phospholipids surrounding an area of water. This structure is called a liposome.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Lipids. (2017). In ScienceAid. Retrieved Apr 28, 2024, from https://scienceaid.net/biology/biochemistry/lipids.html
MLA (Modern Language Association) "Lipids." ScienceAid, scienceaid.net/biology/biochemistry/lipids.html Accessed 28 Apr 2024.
Chicago / Turabian ScienceAid.net. "Lipids." Accessed Apr 28, 2024. https://scienceaid.net/biology/biochemistry/lipids.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Biochemistry
Recent edits by: Jamie (ScienceAid Editor)