Purification of Copper
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
Why Recycle Copper?
The purification of copper is a form of recycling, it is a way of obtaining new, pure copper from old pipes, wires, circuits, and so on. It uses electrolysis.
Process
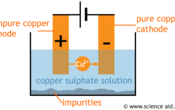
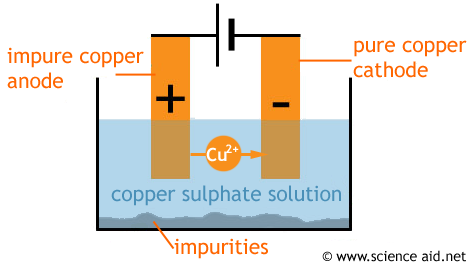
A rod of pure copper is used as a cathode and the impure copper is an anode. The electrolyte (solution the ions travel through) is a solution of copper (II) sulphate (CUSO4).
During electrolysis, copper (II) ions leave the impure copper anode and since they are positive, they are attracted to the negative cathode. Here they pick up the two electrons that are needed to form a copper metal, and this builds up pure copper. Impurities from the anode collect at the bottom of the solution.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Purification of Copper. (2017). In ScienceAid. Retrieved Apr 29, 2024, from https://scienceaid.net/chemistry/applied/copper.html
MLA (Modern Language Association) "Purification of Copper." ScienceAid, scienceaid.net/chemistry/applied/copper.html Accessed 29 Apr 2024.
Chicago / Turabian ScienceAid.net. "Purification of Copper." Accessed Apr 29, 2024. https://scienceaid.net/chemistry/applied/copper.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: Jamie (ScienceAid Editor)