Crude Oil and Fractional Distilation
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
Definition of Crude Oil
Crude oil is the untouched oil as it comes out of the ground. In order to make it useful for a wide range of purposes, it undergoes fractional distillation where the different lengths of alkanes are separated.
Fractional Distillation
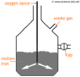
At different levels are bubble caps. The crude oil is heated and the longer chain of alkanes that have a high boiling point will rise out of the bubble cap, but when it reaches its fraction level higher up where it's cooler, the vapour condenses and runs out of the distiller.
Fractions
The groups of oil that are removed at different levels that have similar boiling points are called; fractions.
| Fraction | Uses |
|---|---|
| Gases | A mixture of small hydrocarbons (ethane, propane etc.), liquified as LPG (liquified petroleum gas). This is a chemical feedstock (see below). |
| Petrol | Used as fuel for cars. |
| Kerosene | A fuel for jet engines and oil powered domestic heating. This fraction is used in cracking. |
| Diesel | To power combustion engines in cars and ships. |
| Lubricating Oil | Waxes, greases (like Vaseline) and polishes. Mostly used in cracking. |
| Bitumen | Semi solid tar substance left over from distillation. Used for road surfaces and waterproofing roofs. |
Chemical feedstocks are fractions used to produce various chemicals. Naptha is the chief feedstock and used in paints, cosmetics, drugs, glues and pesticides. This is why oil is so important to us today. Oil isn't just used in cars - and the rapid depletion (loss) of oil reserves is a critical issue.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Crude Oil and Fractional Distilation. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/chemistry/applied/crudeoil.html
MLA (Modern Language Association) "Crude Oil and Fractional Distilation." ScienceAid, scienceaid.net/chemistry/applied/crudeoil.html Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Crude Oil and Fractional Distilation." Accessed Apr 27, 2024. https://scienceaid.net/chemistry/applied/crudeoil.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: Jamie (ScienceAid Editor)