Category:Applied
Applied chemistry looks at how chemistry is used day-to-day.
Titration
Titrations allow you to calculate the concentrations and moles in solutions. The process uses a burette to gradually add one substance to another, an indicator tells you when a reaction point has been reached. See Titration
Testing for Cations
Cations are positive ions such as metal ions (aluminium(III), iron(II), copper(II)). Here we look at two different tests: the flame test and addition of sodium hydroxide. In both color is used to suggest a result. See Testing for Cations
Testing for Anions
Anions are negative ions such as sulphate, carbonate and halogen ions. We test for these using precipitation reactions with barium chloride and silver nitrate. Again, it is the colours of these precipitates that is important. See Testing for Anions
Steel Manufacture
Steel is an alloy of iron, it is produced by removing impurities from iron from the the blast furance by use of an oxygen lance in the basic oxygen steel-making (BOS) process. There are several types including stainless steel. See Steel Manufacture
Solubility of Salts
Some salts are soluble (nitrates, ethanoates), some are not (certain carbonates and hydroxides). The article also explores how they are prepared whether they are soluble or insoluble. See Solubility of Salts
The Haber Process
The Haber Process is the production of ammonia in industry, it is a reversible reaction and so Le Chatelier's principle must be used in balancing the optimum conditions for production. Ammonia is used in fertiliser. See The Haber Process
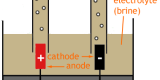
Electrolysis (of Brine)
The process of electrolysis is demonstrated by the electrolysis of brine (NaCl in water), where H+ and Na+ and seperated from OH- and Cl-. The different substances can be tested for and have inductrial applications. See Electrolysis (of Brine)
Crude Oil and Fractional Distillation
Crude oil is the raw material of oil, it is a mixture of different length alkanes, so they must be seperated in fractional distillation. This produces different fractions such as kerosene, gasoline and diesel. See Crude Oil and Fractional Distillation
Purification of Copper
Copper can be recycled by purification. It uses electrolysis with an impure copper anonde and a pure copper cathode. The Copper ions move from the anode to the cathode, leaving impurities at the bottom. See Purification of Copper
The Blast Furnace
The blast furnace is used to extract iron from haematite. Carbon monoxide is the reducing agent and heated air is added to move the reaction. The products from the blast furnace are molten slag and molten air See The Blast Furnace
Extraction of Aluminium
Extraction of aluminium or aluminum is done in a solution of cryolite, this process also uses electrolysis and is very important because of the use of aluminium in the construction of planes and trains. See Extraction of Aluminium
Analysing Gases
A review of the various methods of using and analysing gases in chemistry. Including methods of collection and some tests for identifying various gases including carbon dioxide, sulphur dioxide and ammonia. See Analysing Gases
How to articles in category "Applied"
The following 14 pages are in this category, out of 14 total.