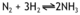Oxidation and Reduction
Edited by Jamie (ScienceAid Editor), SmartyPants
Oxidation and Reduction
The science aid, chemistry category of Industrial Chemistry features many articles that focus on the extraction of metals from their ores, this process involves oxidation and reduction; so this is a very good starting point.
They are both different types of reaction.
Reduction and Oxidation
| Oxidation | The gain of oxygen and the loss of hydrogen and electrons. |
| Reduction | The loss of oxygen and gain of hydrogen and electrons. |
Redox and Metal Ores
A redox reaction is simply one where both oxidation and reduction take place, and the word redox is made up of the first syllable of each word REDuction OXidation.
The extraction of a metal from its ore depends on its reactivity. A metal that is high in the reactivity series (like potassium, calcium) can only be obtained by electrolysis because metals high in the reactivity series form stable compounds. An example of a metal extracted like this is aluminium.
It is metals in the middle of the reactivity series (like zinc and tin) that are extracted by reduction reactions. An example of metal extracted like this is iron.
And metals low in the reactivity series (like gold and platinum) form unstable compounds and can be extracted from their ores simply by heating.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Oxidation and Reduction. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/chemistry/applied/oxidationreduction.html
MLA (Modern Language Association) "Oxidation and Reduction." ScienceAid, scienceaid.net/chemistry/applied/oxidationreduction.html Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Oxidation and Reduction." Accessed Apr 27, 2024. https://scienceaid.net/chemistry/applied/oxidationreduction.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: Jamie (ScienceAid Editor)