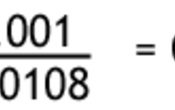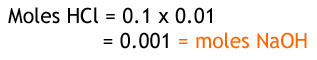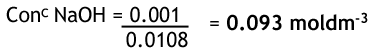Titration
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), nisitha, Jen Moreau and 3 others
The Process
The process of titration is used to calculate the concentration of a solution by neutralizing it and doing some maths (all explained below).
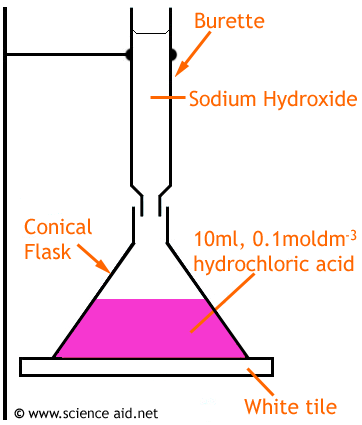
| Measure 10cm3 of acid using a pipette for accuracy. Then release it into the conical flask. | |
| Add a few drops of indicator (in this example, phenolphthalein) to the conical flask | |
| Fill the burette to the top indicator with sodium hydroxide solution | |
| Put the conical flask on top of a white tile, this makes it easier to notice any color change. Open the burette so the NaOH slowly flows/drops into the conical flask. | |
| Swirl the conical flask. Toward the end, the solution turns pink, but when swirled, returns to colorless. Now add the NaOH dropwise. Now turn off the burette as soon as the pink doesn't disappear. | |
| Record the amount of NaOH used. | |
| Repeat the experiment to get concordant (the same) results. |
Calculating Concentrations
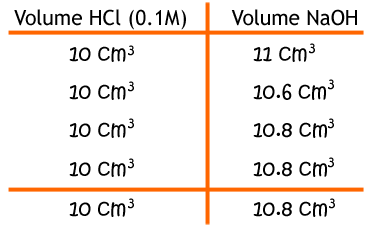
Say that these were your results:
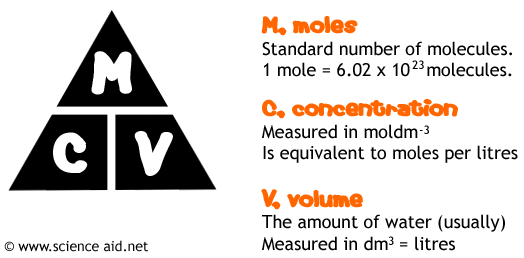
The most important thing to know when doing this calculation is this triangle, all further calculations are based on this.
Now to continue, we need the balanced equation for this reaction
HCl + NaOH ==>> NaCl + H2O
From the reaction, we can see that for every 1 hydrochloric acid there is one sodium hydroxide, so their moles are the same. Given that we have the concentration and volume of HCl, we can work out the number of moles. It is divided by 0.01 since there are 1000 cm3 in 1 dm3.
Now we have the moles of sodium hydroxide and also the volume we can work out the concentration.
Questions and Answers
In the estimation of calcium hardness of water, how to know the volume of EDTA(titrate) consumed from the salt weight 0r molarity of salt?
Without performing the titration experiment how to tell the volume of EDTA consumed from the salt weight. Just to know the answer before the experiment is done. I have tried: Do experiment several numbers of times lack of time not getting a proper result so I need to know the answer before the experiment is done. I think it was caused by Lack of time not getting a proper result so I need to know the answer before the experiment is done
If what you are looking for is a different method to determine the hardness of water without performing experiments or calculations, you can use an Electric Conductivity Meter. It measures the variation in conductivity of water which is proportional to a load of dissolved salts.
If what you want to know is how much EDTA is needed to perform a titration, follow this example:
0.01 M of EDTA solution and ammonia pH 10.0 buffer. Indicator - either in the form of solution, or ground with NaCl -100 mg of indicator plus 20 g of analytical grade NaCl.
Procedure: Transfer exactly 50 mL of water to 250 mL Erlenmayer flask. Acidify the solution with hydrochloric acid. Bring to boil, cool down. Alkalize with ammonia. Filter solution through filter paper. Add 1 ml of pH 10 ammonia buffer. Add 3 drops of Eriochrome Black T solution or pinch of Eriochrome Black T ground with NaCl. Titrate with 0.01M EDTA solution till color changes from violet to blue.
Example methods section for simple acid/base titrations?
Hi Jamie, I'm just digging around trying to find a scientific article with an example of the "Methods" section for a simple titration. Unfortunately, I can't find any suitable papers, only books. Any ideas to direct my search? Thanks, Sam
To get a detailed explanation of an example of a simple titration calculation you can watch this tutorial video by DKhan Academy Organic Chemistry
Or, in case you prefer a written explanation, this is a step by step titration method explanation, which includes the procedure for a titration with an indicator and also, with a PH meter published by Dartmouth College
I need a full lab report on the titration of sodium hydroxide with hydrochloric acid?
Please can you provide me with a template/example of one?
Keep in mind that a full report must include:
- A background
- Purpose
- Indicate precautions
- The terms under which you will be proceeding
- Materials needed
- Detailed procedure
- Observations
- Results.
Follow this example of how a full lab report should be reported, created by Austin Peay State University Department of Chemistry
In case you want to compare it with another full lab report to get a better understanding on how to present one, follow this link to get another example of Titration of Hydrochloric acid with Sodium Hydroxide: presented by Openstudy
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Titration. (2017). In ScienceAid. Retrieved Apr 26, 2024, from https://scienceaid.net/chemistry/applied/titration.html
MLA (Modern Language Association) "Titration." ScienceAid, scienceaid.net/chemistry/applied/titration.html Accessed 26 Apr 2024.
Chicago / Turabian ScienceAid.net. "Titration." Accessed Apr 26, 2024. https://scienceaid.net/chemistry/applied/titration.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Applied
Recent edits by: StephWrites, SmartyPants, Jen Moreau