Mass Spectrometry
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), SarMal, Sharingknowledge
Fundamental particles are the smallest particles that you need to know about in Chemistry. An understanding of these particles is key to understanding the properties of chemicals and how they interact with each other.
Protons, Neutrons and Electrons
| Particle | Relative Charge | Relative Mass |
|---|---|---|
| Proton | +1 | 1 |
| Electron | -1 | 0 (actually 1/1840) |
| Neutron | 0 | 1 |
The electron is important in the structure of the atom because it affects chemical reactions. The nucleons (particles in nucleus: protons and neutrons) are important in the structure because they affect the physical properties.
Other Information
The mass number (A) is the sum of the relative masses of all the particles in the atom. In other words, it is the number of protons and neutrons (or nucleons) in the atom.
The proton number (Z) is fairly obvious. It is the number of protons there are in the atom. It could also be called the atomic number since this is equivalent to the number of electrons in an atom of a certain substance.
Where the name of the element is X, and using the above letters, a shorthand of an element is the following:
An isotope is an element with the same proton number but different mass number: i.e. it has a different number of neutrons. Isotopes can have different physical properties because of their differing masses; for example, the rate of diffusion of some isotopes are unstable and emit radiation.
For the purpose of the calculations also covered in this unit, the mass number is very important, but if we have lots of different isotopes then how can we obtain a precise figure to use? What chemists have to do is calculate the relative atomic mass, which is defined as the mass of an atom, relative to 1/12 of carbon-12. This is a kind of average of all the atomic masses of elements, and this is done using a tool called a mass spectrometer.
Mass Spectrometry
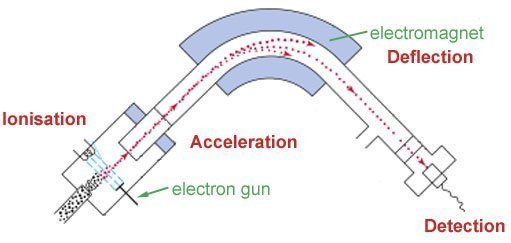
The mass spectrometer is a very important tool for chemists. Below is a diagram of it and underneath are explanations of how each part works.
The Process
- 1The first stage is ionization, where a sample of atoms is injected into the vacuum chamber. An electron gun bombards the sample with electrons knocking some from the atoms, creating cations. If molecules are used they may be broken into fragments. The following reaction is an example of what happens at this stage: Mg(g) ® M+(g) + e-Ionization.Advertisement
- 2Negatively charged plates produce an electric field that accelerates the ions towards the electromagnet.Acceleration.
- 3This takes place next, whereby a magnetic field deflects the ions. The extent of the deflection depends on the mass of the ions. The greater its mass, the less it is deflected. By varying the field strength, different ions will pass through the collection slit.Deflection.
- 4Finally, detection takes place. A tiny current is produced when the cation reaches the detector. A recorder counts the number of signals and represents this as peaks on a mass spectrum graph.Detection.
Interpreting The Graphs
The above graph is the mass spectrum for copper. On the Y (vertical) axis is abundance measured in %, and on the X (horizontal) axis is mass/charge. Which is the mass of the particle divided by the charge.
In order to calculate the RAM (relative atomic mass) of copper based on these results, we need to know the mass of each particle, and then its abundance. Closer to the origin are two peaks at 31.5 and 32.5, and further along are two peaks which are double this. Therefore the 31.5 must be a Cu2+.
To get the RAM - you add the abundances of the different masses (not mass/charge) of the ions and do the following calculation:
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Mass Spectrometry. (2017). In ScienceAid. Retrieved Apr 28, 2024, from https://scienceaid.net/chemistry/fundamental/particles.html
MLA (Modern Language Association) "Mass Spectrometry." ScienceAid, scienceaid.net/chemistry/fundamental/particles.html Accessed 28 Apr 2024.
Chicago / Turabian ScienceAid.net. "Mass Spectrometry." Accessed Apr 28, 2024. https://scienceaid.net/chemistry/fundamental/particles.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Fundamental
Recent edits by: SarMal, Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)