Intermolecular Forces
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
About Intermolecular Forces
Intermolecular bonding is a form of bonding that exists between molecules. The strength of the intermolecular bonds determines how easily the molecules will separate, and hence determine the melting and boiling points. There are different strengths of intermolecular bonds, and we shall look at them in order. Starting with the weakest:
Van der Waals
Also known as induced dipoles and even London Forces. This is a weak force caused by the attraction of temporary dipoles. The diagram below shows how they form.
Electrons are constantly moving about in a random pattern in their energy level. This means that different parts of the molecule carry a very slightly negative charge, known as δδ- (delta delta minus), as it is so small. This movement induces a dipole in neighbouring molecules. Because electrons are always moving around very quickly, the charges also move around. It is also important to note that the more electrons in a molecule / atom, the stronger these Van der Waals, or London forces are. This is seen in the increasing boiling points of the noble gases as you go down the group.
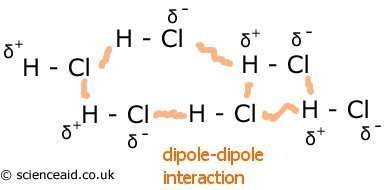
Permanent Dipoles
In bonding, you will have learned about polarization, and how a permanent dipole is produced. In this type of molecular|bonding, the opposite delta charges are attracted. This type of bonding is stronger than the above. Below is an example of permanent dipole interactions in HCl.
Hydrogen Bonding
Hydrogen bonding is much stronger than the previous two and is a special type of permanent dipole-dipole. It only happens to molecules with hydrogen bonds with the following: Oxygen (O), Nitrogen (N) and Fluorine (F). This is because these molecules have high electronegativities (4, 3.5 and 3).
As you can see in the above diagram. When these atoms bond to hydrogen, it leaves lone pairs of electrons that are not being used in bonding. This creates electrostatic forces between molecules. Hydrogen bonds exist in our most abundant molecule on earth: water. This gives it all kinds of properties that are essential for life
.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Intermolecular Forces. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/chemistry/fundamental/inter.html
MLA (Modern Language Association) "Intermolecular Forces." ScienceAid, scienceaid.net/chemistry/fundamental/inter.html Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Intermolecular Forces." Accessed Apr 27, 2024. https://scienceaid.net/chemistry/fundamental/inter.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Fundamental
Recent edits by: Jamie (ScienceAid Editor)