Redox Reactions: Oxidation and Reduction, Oxidation States and Redox Equations
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
Oxidation and Reduction
- 1Oxidation is the loss of electrons or loss of hydrogen and addition of oxygen.Advertisement
- 2'Reduction is the gain of electrons or gain of hydrogen and loss of oxygen.
- 3Remember it with OIL RIG (Oxidation Is Loss, Reduction Is Gain)Redox refers to a reaction where both of these happens.
Oxidation States
The oxidation state of simple ions is simply its charge. For example, the oxidation state of Mg2+ is +2, however, we also assign oxidation states to other compounds and the charge it would have if it were a simple ion and not bonded. In order to work out the oxidation states of some compounds we need to use some rules.
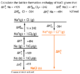
| Oxidation state of an atom in element is 0. | Br in Br2 is 0 |
| The oxidation state of Fluorine is always -1; O is nearly always -2 and Cl is usually -1. | No example. |
| The sum of the oxidation states in polyatomic ions is always the charge on the ion. | In PO43- the Oxygens make -8 so P should be +8 - 3 (the overall charge) meaning its oxidation state here is +5. |
The oxidation gives a compound its name, for example Iron (IV) oxide means the oxidation state of iron is 4 so therefore there must be two oxygen atoms bonded to it. S-block metals loose their electrons in a reaction, so are good reductants (they are oxidized easily). There are also p-block elements which can have different oxidation states; and those in group VII, gain electrons in reactions so are good oxidants (they gain electrons easily).
Redox Equations
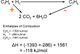
Have a look at the reaction below. Fe2O3 + 2Al ==>> Al2O3 + 2Fe In this reaction iron is reduced (is an oxidant) because it gains electrons and goes from ion to element. The reverse is true for aluminium, which is oxidized (is a reductant). To show this we use half equations as below. Fe3+ + 3e- ==>> Fe Al ==>> Al3</sub>+ + 3e- Another skill you need is to combine half equations. Use the following example where concentrated nitric acid is added to copper. To do this you simply need to have the same number of electrons in each equation and then add combine, the same electrons means they will cancel.
Remember that in acid, you can balance with H+ ions as well.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Redox Reactions: Oxidation and Reduction, Oxidation States and Redox Equations. (2017). In ScienceAid. Retrieved Apr 26, 2024, from https://scienceaid.net/chemistry/physical/redox.html
MLA (Modern Language Association) "Redox Reactions: Oxidation and Reduction, Oxidation States and Redox Equations." ScienceAid, scienceaid.net/chemistry/physical/redox.html Accessed 26 Apr 2024.
Chicago / Turabian ScienceAid.net. "Redox Reactions: Oxidation and Reduction, Oxidation States and Redox Equations." Accessed Apr 26, 2024. https://scienceaid.net/chemistry/physical/redox.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physical
Recent edits by: Jamie (ScienceAid Editor)