States and Shapes
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor)
States of Matter
Please also see bonding for information relating to this.
You must know the difference between solids liquids and gases by now so I won't patronize you, but there is some additional information you need to know. Enthalpy of fusion is the energy needed to loosen forces holding particles together to go from solid to liquid. And enthalpy of vaporization is the energy to overcome the forces holding particles together, making a gas.
As you can see in bonding, there are several different types of crystals. We're going to look at these two for now.
- 1These crystals have low melting and boiling points, are not conductive and have varying solubilities.Molecular Crystals are covalent molecules held together by a form of intermolecular force.Advertisement
- 2They generally have very high melting and boiling points, are not conductive (apart from graphite which conducts electricity) and are insoluble.Macromolecular crystals are huge structures like diamond and graphite.
Shapes of Molecules
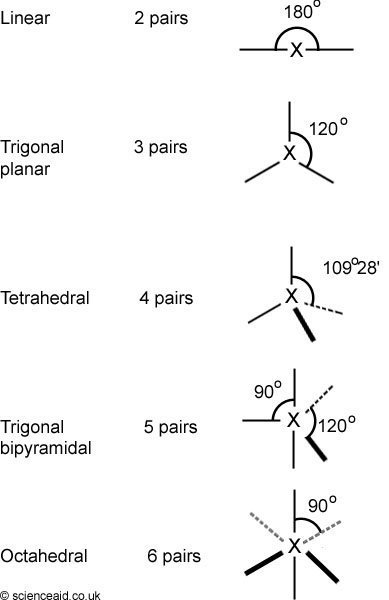
Covalent molecules are made up of pairs of electrons that make a cloud of electrons. It is the repulsion between these pairs that mean different molecules have different shapes. The below diagram shows the generalized shapes of molecules, depending on how many electron pairs there are.
It is not, however, that simple. You have to take into consideration the fact that there are different types of electron pairs. For example,lone pairs are much more repulsive than bonded pairs which are pairs being used in bonds.
Therefore, it logically follows that the repulsion between two lone pairs is strongest; than between a lone and bonded pair - and then two bonded pairs. What all this means is - what the bond angles do in molecules do not comply with the idealized structure in the table above. For example, in water the bond angle between hydrogens is 105o, instead of the standard tetrahedral 109o28' (109 degrees 28 minutes).
Note A 'minute' in geometry is 1/60 (0.167) of a degree.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
States and Shapes. (2017). In ScienceAid. Retrieved Apr 26, 2024, from https://scienceaid.net/chemistry/fundamental/states.html
MLA (Modern Language Association) "States and Shapes." ScienceAid, scienceaid.net/chemistry/fundamental/states.html Accessed 26 Apr 2024.
Chicago / Turabian ScienceAid.net. "States and Shapes." Accessed Apr 26, 2024. https://scienceaid.net/chemistry/fundamental/states.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Fundamental
Recent edits by: Jamie (ScienceAid Editor)