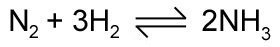Equilibria and Le Chatelier's Principle
Edited by Jamie (ScienceAid Editor)
Reversible Reactions
In most of the reactions we look at they go to completion which means the reactants combine and produce the product/s. However this is not true of all reactions; and such reactions are reversible as in the Haber Process.
The symbol means that the reaction is going in both reactions and is in a state of dynamic equilibrium, the definition for this is that the concentrations of both the reactants and products remains the same.
Le Chatelier's Principle
Le Chatelier [shah - tel - iy - ay] principle says that a system in equilibrium will react to oppose any change placed upon it.
In practice this means the reaction will do the opposite of what is being done. For example, the Haber process reaction is exothermic, so if you heat up the container this reaction is taking place in, less of the forward reaction (which produces heat) will take place so the backward will be more. This is described as shifting the equilibrium to the left.
Now if we look at the effects of pressure. In the Haber reaction there are 4 moles on the left but only two on the right; therefore the pressure is dropping in the forward reaction. So if we increase the pressure the reactions will oppose this change by doing more of the forward reaction which will reduce pressure. Therefore the equilibrium is shifted to the right and the yield of ammonia is improved.
A catalyst doesn't affect the position of equilibrium. If you think of the reaction as going backwards and forwards at the same rates; the catalyst will only make this movement happen quicker which doesn't give you any more of one or the other.
Haber Process Applications
The Haber process is heavily referenced in this article; and like many reactions its purpose is to manufacture ammonia to be sold. Therefore Chemists want to find a way of doing the reaction in such a way that they spend the least amount on making it happen and get the most out of it.
To do this they need to consider the principles of equilibrium. An intermediate temperature of 480°C. But you may think: that isn't low? It is true that the yield of ammonia is better at lower temperatures, however it also means the reaction is slower, so these two issues have to balanced. A similar situation is exists for pressure, since high pressure requires expensive equipment that can withstand the conditions.
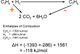
Another industrial reactions that happens in equilibrium is the contact process to make sulphuric acid, which, through using the principles set out in this page, Chemists have got to a 99.5% conversion; with the remaining 0.5% being recycled back into the reaction.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Equilibria and Le Chatelier's Principle. (2017). In ScienceAid. Retrieved Apr 27, 2024, from https://scienceaid.net/chemistry/physical/equilibria.html
MLA (Modern Language Association) "Equilibria and Le Chatelier's Principle." ScienceAid, scienceaid.net/chemistry/physical/equilibria.html Accessed 27 Apr 2024.
Chicago / Turabian ScienceAid.net. "Equilibria and Le Chatelier's Principle." Accessed Apr 27, 2024. https://scienceaid.net/chemistry/physical/equilibria.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Physical