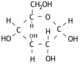Proteins: Amino Acids, Polypeptides, Structures
Edited by Jamie (ScienceAid Editor), Taylor (ScienceAid Editor), Jen Moreau
Proteins are an extremely important type of molecule, making up everything from your finger nails to the haemoglobin that carries oxygen in your blood. Proteins are very important and can be quite complicated in structure.
The Monomer: Amino Acids
For a more detailed look at amino acids, see the chemistry page [chemistry/organic/amines.html|amino acids].
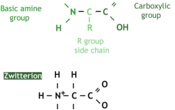
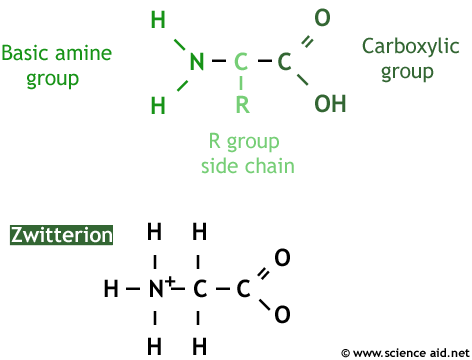
A protein, put simply, is a special type of polymer. And the monomer of it is the amino acid. An amino acid's structure varies depending on the conditions it's in. The first image shows the amino acid as it is in crystallized form, and the image below is how it appears in nature as a zwitterion.
The amino acid is made up of three parts, as identified on the image. The charge of the amino acid changes depending on pH.
| Low pH. | An extra hydrogen on the N so it is positive. |
| Neutral pH. | A zwitterion (both positive and negative parts). |
| High pH. | A hydrogen is lost from the OH so it is negative. |
The R-group means any molecule can replace the 'R'. For instance, glycine simply has an -H instead and alanine a -CH3. This is why there is so much variation in amino acids. In total there are 22 different R-groups, each with a different three-letter abbreviation to represent it (Asn, Glu). Different amino acids are chosen in protein synthesis.
Polypeptides
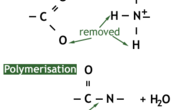
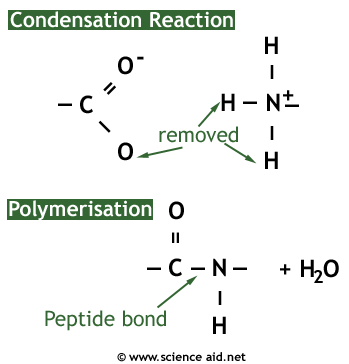
When a number of amino acids join up they are called polypeptides. Two polypeptides are called a dipeptide, three are called a tripeptide. Amino acids form by a condensation reaction as carbohydrates do, producing water as a product and then making a peptide bond. The image below shows graphically how this happens.
Protein Structure
The structure of the protein can be divided into several different levels: primary, secondary, tertiary and quaternary. Below we go through each step-by-step.
- 1The primary structure of the protein relates to the sequence of amino acids, so it isn't really the protein structure, since it does not naturally stay as merely a chain of amino acids. The primary structure is also known as the protein sequence.The Primary Structure.Advertisement
- 2
- 3The protein's tertiary structure is it's overall 3D shape, and involves the R-groups as well. It is made by weak hydrogen bonds, ionic bonds (which are quite strong) and disulphide bridges (between two sulphur atoms, very strong). This is involved in creating the shape of the active site in enzymes.The Tertiary Structure.
- 4And finally there is the quaternary structure. This is where multiple proteins come together to make what appears to be a big mess, but which nonetheless, is very important. For example; haemoglobin, which must have a particular shape so the iron ions can pick up oxygen to carry around the blood stream.Quaternary Structure.
Structure to Function
A globular protein is a bit like a ball (e.g. haemoglobin and immunoglobulin). These are used for transport. They are enzymes and in membranes; since they are fluid (i.e. they can move).
A fibrous protein is long and thin, like a strand or fibre (hence the name). Myosin and collagen are examples of fibrous proteins, used to make muscle and bone. These types of proteins tend to be structural.
Referencing this Article
If you need to reference this article in your work, you can copy-paste the following depending on your required format:
APA (American Psychological Association)
Proteins: Amino Acids, Polypeptides, Structures. (2017). In ScienceAid. Retrieved Apr 29, 2024, from https://scienceaid.net/biology/biochemistry/proteins.html
MLA (Modern Language Association) "Proteins: Amino Acids, Polypeptides, Structures." ScienceAid, scienceaid.net/biology/biochemistry/proteins.html Accessed 29 Apr 2024.
Chicago / Turabian ScienceAid.net. "Proteins: Amino Acids, Polypeptides, Structures." Accessed Apr 29, 2024. https://scienceaid.net/biology/biochemistry/proteins.html.
If you have problems with any of the steps in this article, please ask a question for more help, or post in the comments section below.
Comments
Article Info
Categories : Biochemistry
Recent edits by: Taylor (ScienceAid Editor), Jamie (ScienceAid Editor)